Identification of hair cycle-associated genes from time-course gene expression profile data by using replicate variance
- PMID: 15520371
- PMCID: PMC524696
- DOI: 10.1073/pnas.0407114101
Identification of hair cycle-associated genes from time-course gene expression profile data by using replicate variance
Abstract
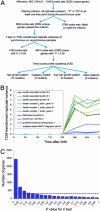
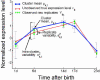
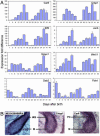
The hair-growth cycle is an example of a cyclic process that is well characterized morphologically but understood incompletely at the molecular level. As an initial step in discovering regulators in hair-follicle morphogenesis and cycling, we used DNA microarrays to profile mRNA expression in mouse back skin from eight representative time points. We developed a statistical algorithm to identify the set of genes expressed within skin that are associated specifically with the hair-growth cycle. The methodology takes advantage of higher replicate variance during asynchronous hair cycles in comparison with synchronous cycles. More than one-third of genes with detectable skin expression showed hair-cycle-related changes in expression, suggesting that many more genes may be associated with the hair-growth cycle than have been identified in the literature. By using a probabilistic clustering algorithm for replicated measurements, these genes were grouped into 30 time-course profile clusters, which fall into four major classes. Distinct genetic pathways were characteristic for the different time-course profile clusters, providing insights into the regulation of hair-follicle cycling and suggesting that this approach is useful for identifying hair follicle regulators. In addition to revealing known hair-related genes, we identified genes that were not previously known to be hair cycle-associated and confirmed their temporal and spatial expression patterns during the hair-growth cycle by quantitative real-time PCR and in situ hybridization. The same computational approach should be generally useful for identifying genes associated with cyclic processes from complex tissues.
Figures

References
-
- Stenn, K. S. & Paus, R. (2001) Physiol. Rev. 81, 449–494. - PubMed
-
- Fuchs, E., Merrill, B. J., Jamora, C. & DasGupta, R. (2001) Dev. Cell 1, 13–25. - PubMed
-
- Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. (2000) Cell 102, 451–461. - PubMed
-
- Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. (2001) Cell 104, 233–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

