Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype
- PMID: 15520858
- PMCID: PMC524226
- DOI: 10.1172/JCI21378
Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype
Abstract
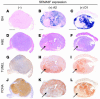
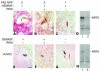
Melanoma is the most lethal skin cancer. Most deaths from melanoma result from metastases. Semaphorins have been shown to inhibit neuronal and endothelial cell migration, but the effects of semaphorins on tumor metastasis have not been documented. We found that semaphorin 3F (SEMA3F) was markedly downregulated in highly metastatic human cell lines in vitro and in vivo, which suggested that it may be a metastasis inhibitor. Metastatic human melanoma cells were transfected with SEMA3F and implanted into mice; the resultant tumors did not metastasize. Rather, the primary tumors resembled benign nevi characterized by large areas of apoptosis, diminished vascularity, inhibition of hyperplasia in overlying epidermal cells, and encapsulated tumor borders delineated by thick layers of fibroblasts and collagen matrix. This phenotype is in stark contrast to highly invasive, vascular mock-transfected tumors. In vitro, tumor cells expressing SEMA3F had a diminished capacity to adhere and migrate on fibronectin. Consistent with semaphorin-mediated chemorepulsion of neurons, tumor cells expressing SEMA3F were chemorepulsive for vascular and lymphatic endothelial cells expressing neuropilin-2 (NRP2), a novel mechanism for a tumor angiogenesis inhibitor. The repulsive activity was abrogated by NRP2 RNA interference. Together these results indicate that SEMA3F is a potent metastasis inhibitor that targets both tumor and stromal cells and raise the possibility of SEMA3F having therapeutic potential.
Figures






References
-
- Kolodkin AL, et al. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. - PubMed
-
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. - PubMed
-
- Harper J, Gerstenfeld LC, Klagsbrun M. Neuropilin-1 expression in osteogenic cells: down-regulation during differentiation of osteoblasts into osteocytes. J. Cell. Biochem. 2001;81:82–92. - PubMed
-
- Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

