Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines
- PMID: 15520866
- PMCID: PMC524232
- DOI: 10.1172/JCI22608
Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines
Abstract
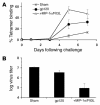
DCs are critical for priming adaptive immune responses to foreign antigens. However, the utility of harnessing these cells in vivo to optimize the immunogenicity of vaccines has not been fully explored. Here we investigate a novel vaccine approach that involves delivering synergistic signals that both recruit and expand DC populations at the site of antigen production. Intramuscular injection of an unadjuvanted HIV-1 envelope (env) DNA vaccine recruited few DCs to the injection site and elicited low-frequency, env-specific immune responses in mice. Coadministration of plasmids encoding the chemokine macrophage inflammatory protein-1alpha (MIP-1alpha) and the DC-specific growth factor fms-like tyrosine kinase 3 ligand with the DNA vaccine resulted in the recruitment, expansion, and activation of large numbers of DCs at the site of inoculation. Consistent with these findings, coadministration of these plasmid cytokines also markedly augmented DNA vaccine---elicited cellular and humoral immune responses and increased protective efficacy against challenge with recombinant vaccinia virus. These data suggest that the availability of mature DCs at the site of inoculation is a critical rate-limiting factor for DNA vaccine immunogenicity. Synergistic recruitment and expansion of DCs in vivo may prove a practical strategy for overcoming this limitation and potentiating immune responses to vaccines as well as other immunotherapeutic strategies.
Figures
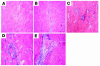

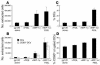
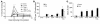
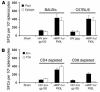

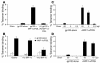
Comment in
-
Developing DNA vaccines that call to dendritic cells.J Clin Invest. 2004 Nov;114(9):1241-4. doi: 10.1172/JCI23467. J Clin Invest. 2004. PMID: 15520855 Free PMC article. Review.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. - PubMed
-
- Wolff JA, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. - PubMed
-
- Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu. Rev. Immunol. 1997;15:617–648. - PubMed
-
- Calarota S, et al. Cellular cytotoxic response induced by DNA vaccination in HIV-1–infected patients. Lancet. 1998;351:1320–1325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

