A novel sensor kinase-response regulator hybrid regulates type III secretion and is required for virulence in Pseudomonas aeruginosa
- PMID: 15522089
- PMCID: PMC3650721
- DOI: 10.1111/j.1365-2958.2004.04331.x
A novel sensor kinase-response regulator hybrid regulates type III secretion and is required for virulence in Pseudomonas aeruginosa
Abstract
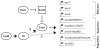
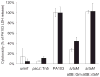
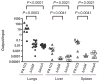
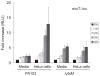
The type III secretion system (TTSS) of Pseudomonas aeruginosa is induced by contact with eukaryotic cells and by growth in low-calcium media. We have identified a protein, RtsM, that is necessary for expression of the TTSS genes in P. aeruginosa. RtsM possesses both histidine kinase and response regulator domains common to two-component signalling proteins, as well as a large predicted periplasmic domain and seven transmembrane domains. Deletion of rtsM resulted in a defect in production and secretion of the type III effectors. Northern blot analysis revealed that mRNAs encoding the effectors ExoT and ExoU are absent in the DeltartsM strain under TTSS-inducing conditions. Using transcriptional fusions, we demonstrated that RtsM is required for transcription of the operons encoding the TTSS effectors and apparatus in response to calcium limitation or to host cell contact. The operon encoding the TTSS regulator ExsA does not respond to calcium limitation, but the basal transcription rate of this operon was lower in deltartsM than in the wild-type parent, PA103. The defect in TTSS effector production and secretion of deltartsM could be complemented by overexpressing ExsA or Vfr, two transcriptional activators involved in TTSS regulation. DeltartsM was markedly less virulent than PA103 in a murine model of acute pneumonia, demonstrating that RtsM is required in vivo. We propose that RtsM is a sensor protein at the start of a signalling cascade that induces expression of the TTSS in response to environmental signals.
Figures



Similar articles
-
Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS.Infect Immun. 2006 Jul;74(7):3880-9. doi: 10.1128/IAI.01891-05. Infect Immun. 2006. PMID: 16790760 Free PMC article.
-
PsrA is a positive transcriptional regulator of the type III secretion system in Pseudomonas aeruginosa.Infect Immun. 2006 Feb;74(2):1121-9. doi: 10.1128/IAI.74.2.1121-1129.2006. Infect Immun. 2006. PMID: 16428760 Free PMC article.
-
Vfr Directly Activates exsA Transcription To Regulate Expression of the Pseudomonas aeruginosa Type III Secretion System.J Bacteriol. 2016 Apr 14;198(9):1442-50. doi: 10.1128/JB.00049-16. Print 2016 May. J Bacteriol. 2016. PMID: 26929300 Free PMC article.
-
Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system.Mol Microbiol. 2006 Nov;62(3):631-40. doi: 10.1111/j.1365-2958.2006.05412.x. Epub 2006 Sep 21. Mol Microbiol. 2006. PMID: 16995895 Review.
-
ExoU is a potent intracellular phospholipase.Mol Microbiol. 2004 Sep;53(5):1279-90. doi: 10.1111/j.1365-2958.2004.04194.x. Mol Microbiol. 2004. PMID: 15387809 Review.
Cited by
-
The RNA Helicase DeaD Stimulates ExsA Translation To Promote Expression of the Pseudomonas aeruginosa Type III Secretion System.J Bacteriol. 2015 Aug;197(16):2664-74. doi: 10.1128/JB.00231-15. Epub 2015 Jun 8. J Bacteriol. 2015. PMID: 26055113 Free PMC article.
-
Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS.Infect Immun. 2006 Jul;74(7):3880-9. doi: 10.1128/IAI.01891-05. Infect Immun. 2006. PMID: 16790760 Free PMC article.
-
Pseudomonas aeruginosa RsmA plays an important role during murine infection by influencing colonization, virulence, persistence, and pulmonary inflammation.Infect Immun. 2008 Feb;76(2):632-8. doi: 10.1128/IAI.01132-07. Epub 2007 Nov 19. Infect Immun. 2008. PMID: 18025099 Free PMC article.
-
A Primed Subpopulation of Bacteria Enables Rapid Expression of the Type 3 Secretion System in Pseudomonas aeruginosa.mBio. 2021 Jun 29;12(3):e0083121. doi: 10.1128/mBio.00831-21. Epub 2021 Jun 22. mBio. 2021. PMID: 34154400 Free PMC article.
-
A conservative amino acid mutation in the master regulator FleQ renders Pseudomonas aeruginosa aflagellate.PLoS One. 2014 May 14;9(5):e97439. doi: 10.1371/journal.pone.0097439. eCollection 2014. PLoS One. 2014. PMID: 24827992 Free PMC article.
References
-
- Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. - PubMed
-
- Becher A, Schweizer HP. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques. 2000;29:948–952. - PubMed
-
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides – SignalP 3.0. J Mol Biol. 2004 (in press) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

