Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: quality-of-life findings: SST report no. 12
- PMID: 15522363
- PMCID: PMC1256021
- DOI: 10.1016/j.ophtha.2004.07.022
Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: quality-of-life findings: SST report no. 12
Abstract
Purpose: To describe health-related quality of life (HRQOL), overall and in patients with unilateral or bilateral choroidal neovascularization (CNV), in a clinical trial (Group N Trial) comparing observation and surgical removal of subfoveal CNV secondary to age-related macular degeneration (AMD).
Design: Randomized clinical trial.
Participants: Eligible patients had untreated subfoveal CNV and AMD, best-corrected visual acuity (VA) of 20/100 to 20/800, classic CNV on fluorescein angiography, and a total subfoveal lesion size of < or =9.0 disc areas in the study eye.
Methods: Health-related quality of life data (the National Eye Institute Visual Function Questionnaire [NEI-VFQ], 36-item Short Form Health Survey [SF-36], and Hospital Anxiety and Depression Scale [HADS]) and clinical data were collected at baseline and at 6, 12, 24, 36, and 48 months. Patients were divided into unilateral and bilateral CNV subgroups based on fluorescein angiographic and clinical evidence.
Main outcome measure: Two-year change in the NEI-VFQ.
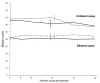
Results: Of 454 patients enrolled, 228 were assigned to observation and 226 to surgery. At baseline, median overall NEI-VFQ scores were 67 in the observation group and 69 in the surgery group; by 2 years, the observation group had lost a median of 3 points (95% confidence interval [CI]: -6 to -2), and the surgery group gained a median of 1 point (CI: -1 to 3). The largest difference was observed for the mental health subscale, where the observation group lost a median of 5 points (CI: -5 to 0), and the surgery group gained a median of 5 points (CI: 0-10) by 2 years. Treatment differences in median 2-year changes in NEI-VFQ scores favored surgery by up to 10 points for unilateral cases and up to 8 points for bilateral cases. No treatment difference in 2-year change was observed for the SF-36 physical component summary; 2-year change in the mental component summary favored surgery by 2 points. Few patients (2%-4%) had HADS definite anxiety or depression at baseline or at 24 months.
Conclusions: Although HRQOL outcomes were better in the submacular surgery arm than in the observation arm, surgery (per protocol) is not recommended because VA outcomes (reported elsewhere) were similar in the treatment arms. This article contains additional online-only material available at http://www.ophsource.com/periodicals/ophtha.
Figures



Comment in
-
Submacular Surgery Trials.Ophthalmology. 2005 Nov;112(11):2055. doi: 10.1016/j.ophtha.2005.08.006. Ophthalmology. 2005. PMID: 16271325 No abstract available.
References
-
- Mangione CM, Berry S, Spritzer K, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998;116:227–33. - PubMed
-
- Mangione CM, Lee PP, Pitts J, et al. NEI-VFQ Field Test Investigators. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) Arch Ophthalmol. 1998;116:1496–504. - PubMed
-
- Mangione CM, Lee PP, Gutierrez PR, et al. National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. - PubMed
-
- Submacular Surgery Trials Research Group. . Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization—SST report no. 1. Arch Ophthalmol. 2003;121:531–9. [correction: 2003;121:1513] - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

