A puromycin-sensitive aminopeptidase is essential for meiosis in Arabidopsis thaliana
- PMID: 15522847
- PMCID: PMC527187
- DOI: 10.1105/tpc.104.024992
A puromycin-sensitive aminopeptidase is essential for meiosis in Arabidopsis thaliana
Abstract
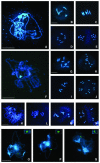
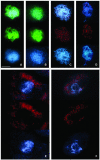
Puromycin-sensitive aminopeptidases (PSAs) participate in a variety of proteolytic events essential for cell growth and viability, and in fertility in a broad range of organisms. We have identified and characterized an Arabidopsis thaliana mutant (mpa1) from a pool of T-DNA tagged lines that lacks PSA activity. This line exhibits reduced fertility, producing shorter siliques (fruits) bearing a lower number of seeds compared with wild-type plants. Cytogenetic characterization of meiosis in the mutant line reveals that both male and female meiosis are defective. In mpa1, early prophase I appears normal, but after pachytene most of the homologous chromosomes are desynaptic, thus, by metaphase I a high level of univalence is observed subsequently leading to abnormal chromosome segregation. Wild-type plants treated with specific inhibitors of PSA show a very similar desynaptic phenotype to that of the mutant line. A fluorescent PSA-specific bioprobe, DAMPAQ-22, reveals that the protein is maximally expressed in wild-type meiocytes during prophase I and is absent in mpa1. Immunolocalization of meiotic proteins showed that the meiotic recombination pathway is disrupted in mpa1. Chromosome pairing and early recombination appears normal, but progression to later stages of recombination and complete synapsis of homologous chromosomes are blocked.
Figures




Similar articles
-
The Arabidopsis thaliana DSB formation (AtDFO) gene is required for meiotic double-strand break formation.Plant J. 2012 Oct;72(2):271-81. doi: 10.1111/j.1365-313X.2012.05075.x. Epub 2012 Jul 26. Plant J. 2012. PMID: 22694475
-
The Arabidopsis MutS homolog AtMSH4 functions at an early step in recombination: evidence for two classes of recombination in Arabidopsis.Genes Dev. 2004 Oct 15;18(20):2557-70. doi: 10.1101/gad.317504. Genes Dev. 2004. PMID: 15489296 Free PMC article.
-
The Arabidopsis MutS homolog AtMSH5 is required for normal meiosis.Cell Res. 2008 May;18(5):589-99. doi: 10.1038/cr.2008.44. Cell Res. 2008. PMID: 18379590
-
Meiotic chromosome synapsis and recombination in Arabidopsis thaliana: new ways of integrating cytological and molecular approaches.Chromosome Res. 2014 Jun;22(2):179-90. doi: 10.1007/s10577-014-9426-8. Chromosome Res. 2014. PMID: 24941912 Review.
-
Coordinating the events of the meiotic prophase.Trends Cell Biol. 2005 Dec;15(12):674-81. doi: 10.1016/j.tcb.2005.10.005. Epub 2005 Oct 27. Trends Cell Biol. 2005. PMID: 16257210 Review.
Cited by
-
Evidence for the Existence in Arabidopsis thaliana of the Proteasome Proteolytic Pathway: ACTIVATION IN RESPONSE TO CADMIUM.J Biol Chem. 2009 Dec 18;284(51):35412-24. doi: 10.1074/jbc.M109.035394. J Biol Chem. 2009. PMID: 19822524 Free PMC article.
-
Mutation of the membrane-associated M1 protease APM1 results in distinct embryonic and seedling developmental defects in Arabidopsis.Plant Cell. 2009 Jun;21(6):1693-721. doi: 10.1105/tpc.108.059634. Epub 2009 Jun 16. Plant Cell. 2009. PMID: 19531600 Free PMC article.
-
A complete chromosome substitution mapping panel reveals genome-wide epistasis in Arabidopsis.Heredity (Edinb). 2024 Sep;133(3):198-205. doi: 10.1038/s41437-024-00705-1. Epub 2024 Jul 9. Heredity (Edinb). 2024. PMID: 38982296 Free PMC article.
-
The role of multifunctional M1 metallopeptidases in cell cycle progression.Ann Bot. 2011 May;107(7):1171-81. doi: 10.1093/aob/mcq265. Epub 2011 Jan 21. Ann Bot. 2011. PMID: 21258033 Free PMC article. Review.
-
Physiological changes and transcript identification in Coreopsis tinctoria Nutt. in early stages of salt stress.PeerJ. 2021 Aug 9;9:e11888. doi: 10.7717/peerj.11888. eCollection 2021. PeerJ. 2021. PMID: 34434650 Free PMC article.
References
-
- Agashe, B., Prasad, C.K., and Siddiqi, I. (2002). Identification and analysis of DYAD: A gene required for meiotic chromosome organisation and female meiotic progression in Arabidopsis. Development 129, 3935–3943. - PubMed
-
- Alexander, M.P. (1969). Differential staining of aborted and nonaborted pollen. Stain Technol. 44, 117–122. - PubMed
-
- Allers, T., and Lichten, M. (2001). Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106, 47–57. - PubMed
-
- Armstrong, S.J., Caryl, A.P., Jones, G.H., and Franklin, F.C.H. (2002). Asy1, a protein required for meiotic chromosome synapsis, localizes to axis-associated chromatin in Arabidopsis and Brassica. J. Cell Sci. 115, 3645–3655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

