Tissue plasminogen activator promotes the effects of corticotropin-releasing factor on the amygdala and anxiety-like behavior
- PMID: 15522965
- PMCID: PMC528975
- DOI: 10.1073/pnas.0407355101
Tissue plasminogen activator promotes the effects of corticotropin-releasing factor on the amygdala and anxiety-like behavior
Abstract
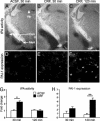
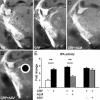
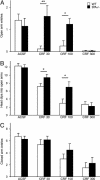
Stress-induced plasticity in the brain requires a precisely orchestrated sequence of cellular events involving novel as well as well known mediators. We have previously demonstrated that tissue plasminogen activator (tPA) in the amygdala promotes stress-induced synaptic plasticity and anxiety-like behavior. Here, we show that tPA activity in the amygdala is up-regulated by a major stress neuromodulator, corticotropin-releasing factor (CRF), acting on CRF type-1 receptors. Compared with WT, tPA-deficient mice responded to CRF treatment with attenuated expression of c-fos (an indicator of neuronal activation) in the central and medial amygdala but had normal c-fos responses in paraventricular nuclei. They exhibited reduced anxiety-like behavior to CRF but had a sustained corticosterone response after CRF administration. This effect of tPA deficiency was not mediated by plasminogen, because plasminogen-deficient mice demonstrated normal behavioral and hormonal changes to CRF. These studies establish tPA as an important mediator of cellular, behavioral, and hormonal responses to CRF.
Figures

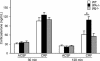
References
-
- Davis, M. (1992) Annu. Rev. Neurosci. 15 353-375. - PubMed
-
- Herman, J. P. & Cullinan, W. E. (1997) Trends Neurosci. 20 78-84. - PubMed
-
- Rogan, M. T. & LeDoux, J. E. (1996) Cell 85 469-475. - PubMed
-
- Owens, M. J. & Nemeroff, C. B. (1991) Pharmacol. Rev. 43 425-473. - PubMed
-
- Vale, W., Spiess, J., Rivier, C. & Rivier, J. (1981) Science 213 1394-1397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

