R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho
- PMID: 15525681
- PMCID: PMC539154
- DOI: 10.1091/mbc.e04-04-0277
R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho
Abstract
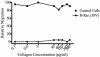
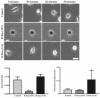
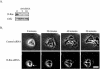
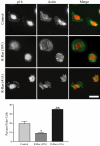
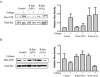
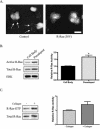
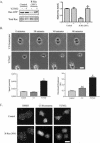
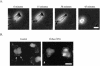
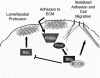
Although it is known that the spatial coordination of Rac and Rho activity is essential for cell migration, the molecular mechanisms regulating these GTPases during migration are unknown. We found that the expression of constitutively activated R-Ras (38V) blocked membrane protrusion and random migration. In contrast, expression of dominant negative R-Ras (41A) enhanced migrational persistence and membrane protrusion. Endogenous R-Ras is necessary for cell migration, as cells that were transfected with siRNA for R-Ras did not migrate. Expression of R-Ras (38V) decreased Rac activity and increased Rho activity around the entire cell periphery, whereas expression of dominant negative R-Ras (41A) showed the converse, suggesting that R-Ras can spatially activate Rho and inactivate Rac. Consistent with this role, endogenous R-Ras localized and was preferentially activated at the leading edge of migratory cells in response to adhesion. The effects of R-Ras on cell migration are mediated by PI3-Kinase, as an effector mutant that uncouples PI3-Kinase binding from R-Ras (38V) rescued migration. From these data, we hypothesize that R-Ras plays a key role in cell migration by locally regulating the switch from Rac to Rho activity after membrane protrusion and adhesion.
Figures


References
-
- Arthur, W. T., Petch, L. A., and Burridge, K. (2000). Integrin engagement suppressed RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719-722. - PubMed
-
- Banyard, J., Anand-Apte, B., Symons, M., and Zetter, B. R. (2000). Motility and invasion are differentially modulated by Rho family GTPases. Oncogene 19, 580-591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

