Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors
- PMID: 15525762
- PMCID: PMC6730255
- DOI: 10.1523/JNEUROSCI.3027-04.2004
Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors
Abstract
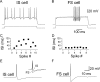
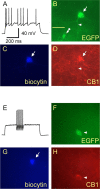
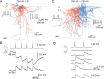
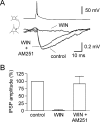
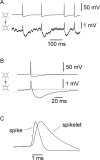
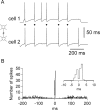
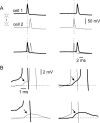
Anatomical studies have shown that the G-protein-coupled cannabinoid receptor-1 (CB1) is selectively expressed in a subset of GABAergic interneurons. It has been proposed that these cells regulate rhythmic activity and play a key role mediating the cognitive actions of marijuana and endogenous cannabinoids. However, the physiology, anatomy, and synaptic connectivity of neocortical CB1-expressing interneurons remain poorly studied. We identified a population of CB1-expressing interneurons in layer II/III in mouse neocortical slices. These cells were multipolar or bitufted, had a widely extending axon, and exhibited a characteristic pattern of irregular spiking (IS) in response to current injection. CB1-expressing-IS (CB1-IS) cells were inhibitory, establishing GABAA receptor-mediated synapses onto pyramidal cells and other CB1-IS cells. Recently, electrical coupling among other classes of cortical interneurons has been shown to contribute to the generation of rhythmic synchronous activity in the neocortex. We therefore tested whether CB1-IS interneurons are interconnected via electrical synapses using paired recordings. We found that 90% (19 of 21 pairs) of simultaneously recorded pairs of CB1-IS cells were electrically coupled. The average coupling coefficient was 6%. Signaling through electrical synapses promoted coordinated firing among CB1-IS cells. Together, our results identify a population of electrically coupled CB1-IS GABAergic interneurons in the neocortex that share a unique morphology and a characteristic pattern of irregular spiking in response to current injection. The synaptic interactions of these cells may play an important role mediating the cognitive actions of cannabinoids and regulating coherent neocortical activity.
Figures


References
-
- Beierlein M, Gibson JR, Connors BW (2000) A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci 3: 904-910. - PubMed
-
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H (2003) A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron 38: 805-817. - PubMed
-
- Bodor AL, Katona I, Mackie K, Hajos N, Freund TF (2003) Distinct types of interneurons are targeted by endocannabinoids in the rat soomatosensory cortex. Soc Neurosci Abstr 29: 578.13.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
