Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism
- PMID: 15525768
- PMCID: PMC6730228
- DOI: 10.1523/JNEUROSCI.2557-04.2004
Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism
Abstract
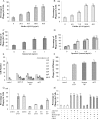
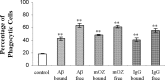
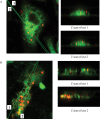
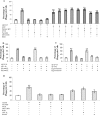
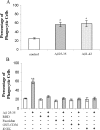
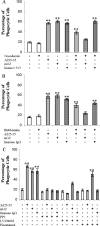
Microglia are the principle immune effector and phagocytic cells in the CNS. These cells are associated with fibrillar beta-amyloid (fAbeta)-containing plaques found in the brains of Alzheimer's disease (AD) patients. The plaque-associated microglia undergo a phenotypic conversion into an activated phenotype and are responsible for the development of a focal inflammatory response that exacerbates and accelerates the disease process. Paradoxically, despite the presence of abundant activated microglia in the brain of AD patients, these cells fail to mount a phagocytic response to Abeta deposits but can efficiently phagocytose Abeta fibrils and plaques in vitro. We report that exposure of microglia to fAbeta in vitro induces phagocytosis through mechanisms distinct from those used by the classical phagocytic receptors, the Ig receptors (FcRgammaI and FcgammaRIII) or complement receptors. Microglia interact with fAbeta through a recently characterized Abeta cell surface receptor complex comprising the B-class scavenger receptor CD36, alpha6beta1 integrin, and CD47 (integrin-associated protein). Antagonists specific for each component of the receptor complex blocks fAbeta-stimulated phagocytosis. These data demonstrated that engagement of this ensemble of receptors is required for induction of phagocytosis. The phagocytic response stimulated by this receptor complex is driven principally by a beta(1) integrin-linked process that is morphologically and mechanistically distinct from the classical type I and type II phagocytic mechanisms. These data provide evidence for phagocytic uptake of fAbeta through a receptor-mediated, nonclassical phagocytic mechanism.
Figures

References
-
- Aderem A, Underhill DM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17: 593-623. - PubMed
-
- Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD (1996) Scavenging of Alzheimer's amyloid beta-protein by microglia in culture. J Neurosci Res 43: 190-202. - PubMed
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T (2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916-919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
