Transcranial direct current stimulation during sleep improves declarative memory
- PMID: 15525784
- PMCID: PMC6730231
- DOI: 10.1523/JNEUROSCI.2725-04.2004
Transcranial direct current stimulation during sleep improves declarative memory
Erratum in
- J Neurosci. 2005 Jan 12;25(2):1 p following 531
Abstract
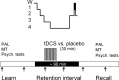
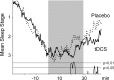
In humans, weak transcranial direct current stimulation (tDCS) modulates excitability in the motor, visual, and prefrontal cortex. Periods rich in slow-wave sleep (SWS) not only facilitate the consolidation of declarative memories, but in humans, SWS is also accompanied by a pronounced endogenous transcortical DC potential shift of negative polarity over frontocortical areas. To experimentally induce widespread extracellular negative DC potentials, we applied anodal tDCS (0.26 mA) [correction] repeatedly (over 30 min) bilaterally at frontocortical electrode sites during a retention period rich in SWS. Retention of declarative memories (word pairs) and also nondeclarative memories (mirror tracing skills) learned previously was tested after this period and compared with retention performance after placebo stimulation as well as after retention intervals of wakefulness. Compared with placebo stimulation, anodal tDCS during SWS-rich sleep distinctly increased the retention of word pairs (p < 0.005). When applied during the wake retention interval, tDCS did not affect declarative memory. Procedural memory was also not affected by tDCS. Mood was improved both after tDCS during sleep and during wake intervals. tDCS increased sleep depth toward the end of the stimulation period, whereas the average power in the faster frequency bands (,alpha, and beta) was reduced. Acutely, anodal tDCS increased slow oscillatory activity <3 Hz. We conclude that effects of tDCS involve enhanced generation of slow oscillatory EEG activity considered to facilitate processes of neuronal plasticity. Shifts in extracellular ionic concentration in frontocortical tissue (expressed as negative DC potentials during SWS) may facilitate sleep-dependent consolidation of declarative memories.
Figures



References
-
- Achermann P, Borbely AA (1997) Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 81: 213-222. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
