p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death
- PMID: 15525786
- PMCID: PMC6730234
- DOI: 10.1523/JNEUROSCI.2114-04.2004
p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death
Abstract
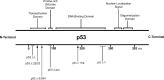
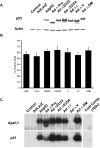
The p53 tumor suppressor gene has been implicated in the regulation of apoptosis in a number of different neuronal death paradigms. Because of the importance of p53 in neuronal injury, we questioned the mechanism underlying p53-mediated apoptosis in neurons. Using adenoviral-mediated gene delivery, reconstitution experiments, and mice carrying a knock-in mutation in the endogenous p53 gene, we show that the transactivation function of p53 is essential to induce neuronal cell death. Although p53 possesses two transactivation domains that can activate p53 targets independently, we demonstrate that the first activation domain (ADI) is required to drive apoptosis after neuronal injury. Furthermore, the BH3-only proteins Noxa and PUMA exhibit differential regulation by the two transactivation domains. Here, we show that Noxa can be induced by either activation domain, whereas PUMA induction requires both activation domains to be intact. Unlike Noxa, the upregulation of PUMA alone is sufficient to induce neuronal cell death. We demonstrate, therefore, that the first transactivation domain of p53 is indispensable for the induction of neuronal cell death.
Figures








References
-
- Banasiak KJ, Haddad GG (1998) Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res 797: 295-304. - PubMed
-
- Baptiste N, Friedlander P, Chen X, Prives C (2002) The proline-rich domain of p53 is required for cooperation with anti-neoplastic agents to promote apoptosis of tumor cells. Oncogene 21: 9-21. - PubMed
-
- Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P (1998) Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 282: 290-293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
