Endocannabinoids link feeding state and auditory perception-related gene expression
- PMID: 15525787
- PMCID: PMC6730237
- DOI: 10.1523/JNEUROSCI.3298-04.2004
Endocannabinoids link feeding state and auditory perception-related gene expression
Abstract
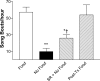
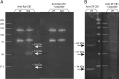
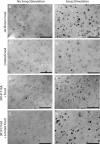
Singing by adult male zebra finches is a learned behavior important for courtship, kin recognition, and nest defense (Zann, 1996) and is inhibited by both brief periods of limited food availability and systemic injection of cannabinoids. These similar effects on singing, combined with increasing evidence for endocannabinoid involvement in feeding behavior, led us to evaluate a possible shared mechanism. We found that limited food availability both reduces singing in a cannabinoid antagonist-reversible manner and increases levels of the endocannabinoid 2-arachidonyl glycerol in various brain regions including the caudal telencephalon, an area that contains auditory telencephalon including the L2 subfield of L (L2) and caudal medial nidopallium (NCM). Development and use of an anti-zebra finch cannabinoid receptor type 1 (CB1) antibody demonstrates distinct, dense cannabinoid receptor expression within song regions including Area X, lMAN (lateral magnocellular nucleus of anterior nidopallium), HVC, RA (robust nucleus of arcopallium), and L2. NCM receives L2 projections and is implicated in integration of auditory information. Activity in this area, determined through expression of the transcription factor ZENK, is increased after exposure to unfamiliar song. Because previous work has shown that these novel song-stimulated increases in NCM activity are mitigated by cannabinoid exposure, we tested and found that similar effects on ZENK expression are produced by limiting food. Limited food-related reductions in the activity of NCM neurons were reversed by the cannabinoid antagonist SR141716A (N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide), implicating CB1 cannabinoid receptor involvement. Taken together, these experiments indicate a link between feeding state and gene expression related to auditory perception that is mediated by endocannabinoid signaling.
Figures




References
-
- Berry EM, Mechoulam R (2002) Tetrahydrocannabinol and endocannabinoids in feeding and appetite. Pharmacol Ther 95: 185-190. - PubMed
-
- Bonke BA, Bonke D, Scheich H (1979) Connectivity of the auditory forebrain nuclei in the guinea fowl (Numida meleagris). Cell Tissue Res 200: 101-121. - PubMed
-
- Clayton DF (2000) The genomic action potential. Neurobiol Learn Mem 74: 185-216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
