A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction
- PMID: 15525788
- PMCID: PMC6730248
- DOI: 10.1523/JNEUROSCI.2034-04.2004
A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction
Abstract
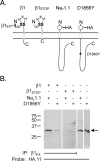
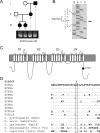
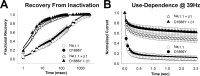
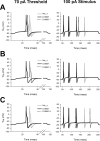
A mutation in the sodium channel SCN1A was identified in a small Italian family with dominantly inherited generalized epilepsy with febrile seizures plus (GEFS+). The mutation, D1866Y, alters an evolutionarily conserved aspartate residue in the C-terminal cytoplasmic domain of the sodium channel alpha subunit. The mutation decreased modulation of the alpha subunit by beta1, which normally causes a negative shift in the voltage dependence of inactivation in oocytes. There was less of a shift with the mutant channel, resulting in a 10 mV difference between the wild-type and mutant channels in the presence of beta1. This shift increased the magnitude of the window current, which resulted in more persistent current during a voltage ramp. Computational analysis suggests that neurons expressing the mutant channels will fire an action potential with a shorter onset delay in response to a threshold current injection, and that they will fire multiple action potentials with a shorter interspike interval at a higher input stimulus. These results suggest a causal relationship between a positive shift in the voltage dependence of sodium channel inactivation and spontaneous seizure activity. Direct interaction between the cytoplasmic C-terminal domain of the wild-type alpha subunit with the beta1 or beta3 subunit was first demonstrated by yeast two-hybrid analysis. The SCN1A peptide K1846-R1886 is sufficient for beta subunit interaction. Coimmunoprecipitation from transfected mammalian cells confirmed the interaction between the C-terminal domains of the alpha and beta1 subunits. The D1866Y mutation weakens this interaction, demonstrating a novel molecular mechanism leading to seizure susceptibility.
Figures


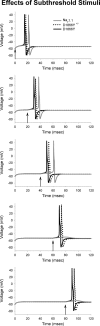
References
-
- Abou-Khalil B, Ge Q, Desai R, Ryther R, Bazyk A, Bailey R, Haines JL, Sutcliffe JS, George JR AL (2001) Partial and generalized epilepsy with febrile seizures plus and a novel SCN1A mutation. Neurology 57: 2265-2272. - PubMed
-
- Abriel H, Cabo C, Wehrens XHT, Rivolta I, Motoike HK, Memmi M, Napolitano C, Priori SG, Kass RS (2001) Novel arrhythmogenic mechanism revealed by a long-QT syndrome mutation in the cardiac Na+ channel. Circ Res 88: 740-745. - PubMed
-
- An R-H, Wang XL, Kerem B, Benhorin J, Medina A, Goldmit M, Kass RS (1998) Novel LQT-3 mutation affects Na+ channel activity through interactions between α- and β1-subunits. Circ Res 83: 141-146. - PubMed
-
- Annesi G, Gambardella A, Carrideo S, Incorpora G, Labate A, Pasqua AA, Civitelli D, Polizzi A, Annesi F, Spadafora P, Tarantino P, Cirò Candiano IC, Romeo N, De Marco EV, Ventura P, LePiane E, Zappia M, Aguglia U, Pavone L, Quattrone A (2003) Two novel SCN1A missense mutations in generalized epilepsy with febrile seizures plus. Epilepsia 44: 1257-1258. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1987) Current protocols in molecular biology. New York: Greene and Wiley.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- GM07767/GM/NIGMS NIH HHS/United States
- T32 RR07008/RR/NCRR NIH HHS/United States
- NS34509/NS/NINDS NIH HHS/United States
- NS38580/NS/NINDS NIH HHS/United States
- R01 NS048336/NS/NINDS NIH HHS/United States
- T32 RR007008/RR/NCRR NIH HHS/United States
- NS26729/NS/NINDS NIH HHS/United States
- NS48336/NS/NINDS NIH HHS/United States
- R01 NS034509/NS/NINDS NIH HHS/United States
- F31 NS043067/NS/NINDS NIH HHS/United States
- MH59980/MH/NIMH NIH HHS/United States
- R01 MH059980/MH/NIMH NIH HHS/United States
- NS43067/NS/NINDS NIH HHS/United States
- R01 NS038580/NS/NINDS NIH HHS/United States
- T32 GM007767/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
