Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons
- PMID: 15525813
- PMCID: PMC2838234
- DOI: 10.1152/jn.00269.2004
Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons
Abstract
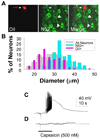
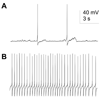
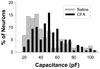
Several painful conditions, including temporomandibular disorders (TMD), are more prevalent and more severe in women than in men. Although the physiological basis for this sex difference remains to be determined, it is likely that estrogen is an underlying factor. The present study was performed to test the hypotheses that estrogen increases the excitability of rat temporomandibular joint (TMJ) afferents and exacerbates the inflammation-induced sensitization of these sensory neurons. Retrogradely labeled TMJ neurons from ovariectomized rats and ovariectomized rats receiving chronic estrogen replacement were studied using whole cell patch-clamp techniques three days after injecting the TMJ with either saline or Complete Freund's Adjuvant to induce inflammation. Excitability was assessed with depolarizing current injection to determine action potential threshold, rheobase, and the response to suprathreshold stimuli. Spontaneous activity was also assessed. Both inflammation and estrogen increased the excitability of TMJ neurons as reflected by decreases in action potential threshold and rheobase and increases in the incidence of spontaneous activity. The effects were additive with neurons from rats receiving both estrogen and inflammation being the most excitable. The increases in excitability were associated with changes in passive properties and action potential waveform, suggesting that estrogen and inflammation affect the expression and/or properties of ion channels in TMJ neurons. Importantly, the influence of estrogen on both baseline and inflammation-induced changes in TMJ neuronal excitability may help explain the profound sex difference observed in TMD as well as suggest a novel target for the treatment of this pain condition.
Figures


References
-
- Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. - PubMed
-
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999a;82:2649–2656. - PubMed
-
- Andrew D, Greenspan JD. Peripheral coding of tonic mechanical cutaneous pain: comparison of nociceptor activity in rat and human psychophysics. J Neurophysiol. 1999b;82:2641–2648. - PubMed
-
- Bar KJ, Schaible HG, Brauer R, Halbhuber KJ, von Banchet GS. The proportion of TRPV1 protein-positive lumbar DRG neurones does not increase in the course of acute and chronic antigen-induced arthritis in the knee joint of the rat. Neurosci Lett. 2004;361:172–175. - PubMed
-
- Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

