AIF deficiency compromises oxidative phosphorylation
- PMID: 15526035
- PMCID: PMC533047
- DOI: 10.1038/sj.emboj.7600461
AIF deficiency compromises oxidative phosphorylation
Abstract
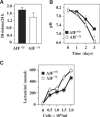
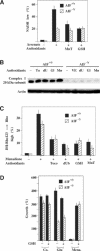
Apoptosis-inducing factor (AIF) is a mitochondrial flavoprotein that, after apoptosis induction, translocates to the nucleus where it participates in apoptotic chromatinolysis. Here, we show that human or mouse cells lacking AIF as a result of homologous recombination or small interfering RNA exhibit high lactate production and enhanced dependency on glycolytic ATP generation, due to severe reduction of respiratory chain complex I activity. Although AIF itself is not a part of complex I, AIF-deficient cells exhibit a reduced content of complex I and of its components, pointing to a role of AIF in the biogenesis and/or maintenance of this polyprotein complex. Harlequin mice with reduced AIF expression due to a retroviral insertion into the AIF gene also manifest a reduced oxidative phosphorylation (OXPHOS) in the retina and in the brain, correlating with reduced expression of complex I subunits, retinal degeneration, and neuronal defects. Altogether, these data point to a role of AIF in OXPHOS and emphasize the dual role of AIF in life and death.
Figures





References
-
- Bénit P, Lebon S, Chol M, Giurgea I, Rötig A, Rustin P (2003) Mitochondrial NADH oxidation deficiency in humans. Curr Genomics 5: 137–146
-
- Budde SM, van den Heuvel LP, Smeets RJ, Skladal D, Mayr JA, Boelen C, Petruzzella V, Papa S, Smeitink JA (2003) Clinical heterogeneity in patients with mutations in the NDUFS4 gene of mitochondrial complex I. J Inherit Metab Dis 26: 813–815 - PubMed
-
- Budijardjo I, Oliver H, Lutter M, Luo X, Wang X (1999) Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15: 269–290 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

