Chemical validation of GPI biosynthesis as a drug target against African sleeping sickness
- PMID: 15526036
- PMCID: PMC533043
- DOI: 10.1038/sj.emboj.7600456
Chemical validation of GPI biosynthesis as a drug target against African sleeping sickness
Abstract
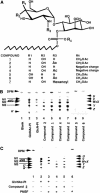
It has been suggested that compounds affecting glycosylphosphatidylinositol (GPI) biosynthesis in bloodstream form Trypanosoma brucei should be trypanocidal. We describe cell-permeable analogues of a GPI intermediate that are toxic to this parasite but not to human cells. These analogues are metabolized by the T. brucei GPI pathway, but not by the human pathway. Closely related nonmetabolizable analogues have no trypanocidal activity. This represents the first direct chemical validation of the GPI biosynthetic pathway as a drug target against African human sleeping sickness. The results should stimulate further inhibitor design and synthesis and encourage the search for inhibitors in natural product and synthetic compound libraries.
Figures



Similar articles
-
Designing sleeping sickness control.ACS Chem Biol. 2008 Oct 17;3(10):601-3. doi: 10.1021/cb800239p. ACS Chem Biol. 2008. PMID: 18928247
-
Probing enzymes late in the trypanosomal glycosylphosphatidylinositol biosynthetic pathway with synthetic glycosylphosphatidylinositol analogues.ACS Chem Biol. 2008 Oct 17;3(10):625-34. doi: 10.1021/cb800143w. ACS Chem Biol. 2008. PMID: 18928250
-
Identification of novel inhibitors of UDP-Glc 4'-epimerase, a validated drug target for african sleeping sickness.Bioorg Med Chem Lett. 2006 Nov 15;16(22):5744-7. doi: 10.1016/j.bmcl.2006.08.091. Epub 2006 Sep 7. Bioorg Med Chem Lett. 2006. PMID: 16962325
-
Glycosyl phosphatidylinositols in Trypanosoma brucei.Exp Parasitol. 1993 Mar;76(2):216-20. doi: 10.1006/expr.1993.1026. Exp Parasitol. 1993. PMID: 8454032 Review. No abstract available.
-
Current chemotherapy of human African trypanosomiasis.Parasitol Res. 2003 Jun;90 Supp 1:S10-3. doi: 10.1007/s00436-002-0752-y. Epub 2002 Nov 23. Parasitol Res. 2003. PMID: 12811544 Review.
Cited by
-
Inhibitors incorporating zinc-binding groups target the GlcNAc-PI de-N-acetylase in Trypanosoma brucei, the causative agent of African sleeping sickness.Chem Biol Drug Des. 2012 Mar;79(3):270-8. doi: 10.1111/j.1747-0285.2011.01300.x. Chem Biol Drug Des. 2012. PMID: 22222041 Free PMC article.
-
Fragment screening reveals salicylic hydroxamic acid as an inhibitor of Trypanosoma brucei GPI GlcNAc-PI de-N-acetylase.Carbohydr Res. 2014 Mar 31;387(100):54-8. doi: 10.1016/j.carres.2013.12.016. Epub 2013 Dec 30. Carbohydr Res. 2014. PMID: 24589444 Free PMC article.
-
Structure and Function of the Glycosylphosphatidylinositol Transamidase, a Transmembrane Complex Catalyzing GPI Anchoring of Proteins.Subcell Biochem. 2024;104:425-458. doi: 10.1007/978-3-031-58843-3_16. Subcell Biochem. 2024. PMID: 38963495 Review.
-
The glycosylphosphatidylinositol (GPI) biosynthetic pathway of bloodstream-form Trypanosoma brucei is dependent on the de novo synthesis of inositol.Mol Microbiol. 2006 Jul;61(1):89-105. doi: 10.1111/j.1365-2958.2006.05216.x. Mol Microbiol. 2006. PMID: 16824097 Free PMC article.
-
Molecular insights into biogenesis of glycosylphosphatidylinositol anchor proteins.Nat Commun. 2022 May 12;13(1):2617. doi: 10.1038/s41467-022-30250-6. Nat Commun. 2022. PMID: 35551457 Free PMC article.
References
-
- Chang T, Milne KG, Güther ML, Smith TK, Ferguson MAJ (2002) Cloning of Trypanosoma brucei and Leishmania major genes encoding the GlcNAc-phosphatidylinositol de-N-acetylase of glycosylphosphatidylinositol biosynthesis that is essential to the African sleeping sickness parasite. J Biol Chem 277: 50176–50182 - PubMed
-
- Cottaz S, Brimacombe JS, Ferguson MAJ (1993) Parasite glycoconjugates. Part 1. Synthesis of some early and related intermediates in the biosynthetic pathway of glycosyl-phosphatidylinositol membrane anchors. J Chem Soc Perkin Trans 1: 2945–2951
-
- Cross GAM (1996) Antigenic variation in trypanosomes: secrets surface slowly. BioEssays 18: 283–287 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

