The molecular basis for oat intolerance in patients with celiac disease
- PMID: 15526039
- PMCID: PMC523824
- DOI: 10.1371/journal.pmed.0010001
The molecular basis for oat intolerance in patients with celiac disease
Abstract
Background: Celiac disease is a small intestinal inflammatory disorder characterized by malabsorption, nutrient deficiency, and a range of clinical manifestations. It is caused by an inappropriate immune response to dietary gluten and is treated with a gluten-free diet. Recent feeding studies have indicated oats to be safe for celiac disease patients, and oats are now often included in the celiac disease diet. This study aimed to investigate whether oat intolerance exists in celiac disease and to characterize the cells and processes underlying this intolerance.
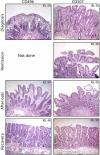
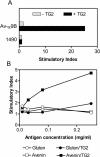
Methods and findings: We selected for study nine adults with celiac disease who had a history of oats exposure. Four of the patients had clinical symptoms on an oats-containing diet, and three of these four patients had intestinal inflammation typical of celiac disease at the time of oats exposure. We established oats-avenin-specific and -reactive intestinal T-cell lines from these three patients, as well as from two other patients who appeared to tolerate oats. The avenin-reactive T-cell lines recognized avenin peptides in the context of HLA-DQ2. These peptides have sequences rich in proline and glutamine residues closely resembling wheat gluten epitopes. Deamidation (glutamine-->glutamic acid conversion) by tissue transglutaminase was involved in the avenin epitope formation.
Conclusions: We conclude that some celiac disease patients have avenin-reactive mucosal T-cells that can cause mucosal inflammation. Oat intolerance may be a reason for villous atrophy and inflammation in patients with celiac disease who are eating oats but otherwise are adhering to a strict gluten-free diet. Clinical follow-up of celiac disease patients eating oats is advisable.
Conflict of interest statement
Figures



Similar articles
-
Ingestion of oats and barley in patients with celiac disease mobilizes cross-reactive T cells activated by avenin peptides and immuno-dominant hordein peptides.J Autoimmun. 2015 Jan;56:56-65. doi: 10.1016/j.jaut.2014.10.003. Epub 2014 Nov 1. J Autoimmun. 2015. PMID: 25457306
-
Immunologic evidence of no harmful effect of oats in celiac disease.Am J Clin Nutr. 2001 Jul;74(1):137-40. doi: 10.1093/ajcn/74.1.137. Am J Clin Nutr. 2001. PMID: 11451729
-
Oats as a Safe Alternative to Triticeae Cereals for People Suffering from Celiac Disease? A Review.Plant Foods Hum Nutr. 2020 Jun;75(2):131-141. doi: 10.1007/s11130-020-00800-8. Plant Foods Hum Nutr. 2020. PMID: 32133597 Review.
-
Purified oat protein can trigger acute symptoms linked to immune activation in coeliac disease patients but not histological deterioration.Gut. 2025 May 7;74(6):906-917. doi: 10.1136/gutjnl-2024-333589. Gut. 2025. PMID: 39961645
-
Why Oats Are Safe and Healthy for Celiac Disease Patients.Med Sci (Basel). 2016 Nov 26;4(4):21. doi: 10.3390/medsci4040021. Med Sci (Basel). 2016. PMID: 29083384 Free PMC article. Review.
Cited by
-
The gluten-free diet and its current application in coeliac disease and dermatitis herpetiformis.United European Gastroenterol J. 2015 Apr;3(2):121-35. doi: 10.1177/2050640614559263. United European Gastroenterol J. 2015. PMID: 25922672 Free PMC article. Review.
-
Intestinal T cell responses to cereal proteins in celiac disease.Dig Dis Sci. 2006 Jan;51(1):202-9. doi: 10.1007/s10620-006-3108-0. Dig Dis Sci. 2006. PMID: 16416236
-
Gluten Immunogenic Peptides as Standard for the Evaluation of Potential Harmful Prolamin Content in Food and Human Specimen.Nutrients. 2018 Dec 5;10(12):1927. doi: 10.3390/nu10121927. Nutrients. 2018. PMID: 30563126 Free PMC article. Review.
-
Oats in the diet of children with celiac disease: preliminary results of a double-blind, randomized, placebo-controlled multicenter Italian study.Nutrients. 2013 Nov 20;5(11):4653-64. doi: 10.3390/nu5114653. Nutrients. 2013. PMID: 24264227 Free PMC article. Clinical Trial.
-
Toward the assessment of food toxicity for celiac patients: characterization of monoclonal antibodies to a main immunogenic gluten peptide.PLoS One. 2008 May 28;3(5):e2294. doi: 10.1371/journal.pone.0002294. PLoS One. 2008. PMID: 18509534 Free PMC article.
References
-
- Sollid LM. Coeliac disease: Dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. - PubMed
-
- Janatuinen EK, Pikkarainen PH, Kemppainen TA, Kosma VM, Jarvinen RM, et al. A comparison of diets with and without oats in adults with celiac disease. N Engl J Med. 1995;333:1033–1037. - PubMed
-
- Hardman CM, Garioch JJ, Leonard JN, Thomas HJ, Walker MM, et al. Absence of toxicity of oats in patients with dermatitis herpetiformis. N Engl J Med. 1997;337:1884–1887. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

