Gelsolin binds to polyphosphoinositide-free lipid vesicles and simultaneously to actin microfilaments
- PMID: 15527423
- PMCID: PMC1134765
- DOI: 10.1042/BJ20041054
Gelsolin binds to polyphosphoinositide-free lipid vesicles and simultaneously to actin microfilaments
Abstract
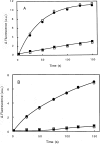
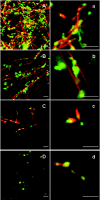
Gelsolin is a calcium-, pH- and lipid-dependent actin filament severing/capping protein whose main function is to regulate the assembly state of the actin cytoskeleton. Gelsolin is associated with membranes in cells, and it is generally assumed that this interaction is mediated by PPIs (polyphosphoinositides), since an interaction with these lipids has been characterized in vitro. We demonstrate that non-PPI lipids also bind gelsolin, especially at low pH. The data suggest further that gelsolin becomes partially buried in the lipid bilayer under mildly acidic conditions, in a manner that is not dependent of the presence of PPIs. Our data also suggest that lipid binding involves a number of sites that are spread throughout the gelsolin molecule. Linker regions between gelsolin domains have been implicated by other work, notably the linker between G1 and G2 (gelsolin domains 1 and 2 respectively), and we postulate that the linker region between the N-terminal and C-terminal halves of gelsolin (between G3 and G4) is also involved in the interaction with lipids. This region is compatible with other studies in which additional binding sites have been located within G4-6. The lipid-gelsolin interactions reported in the present paper are not calcium-dependent, and are likely to involve significant conformational changes to the gelsolin molecule, as the chymotryptic digest pattern is altered by the presence of lipids under our conditions. We also report that vesicle-bound gelsolin is capable of binding to actin filaments, presumably through barbed end capping. Gelsolin bound to vesicles can nucleate actin assembly, but is less active in severing microfilaments.
Figures







Similar articles
-
CapZ-lipid membrane interactions: a computer analysis.Theor Biol Med Model. 2006 Aug 16;3:30. doi: 10.1186/1742-4682-3-30. Theor Biol Med Model. 2006. PMID: 16914033 Free PMC article.
-
Distinct roles of four gelsolin-like domains of Caenorhabditis elegans gelsolin-like protein-1 in actin filament severing, barbed end capping, and phosphoinositide binding.Biochemistry. 2010 May 25;49(20):4349-60. doi: 10.1021/bi100215b. Biochemistry. 2010. PMID: 20392036 Free PMC article.
-
Probing the effects of calcium on gelsolin.Biochemistry. 1997 Dec 16;36(50):15848-55. doi: 10.1021/bi972192p. Biochemistry. 1997. PMID: 9398317
-
Gelsolin: the tail of a molecular gymnast.Cytoskeleton (Hoboken). 2013 Jul;70(7):360-84. doi: 10.1002/cm.21117. Epub 2013 Jun 27. Cytoskeleton (Hoboken). 2013. PMID: 23749648 Review.
-
Effects of cadmium on the actin cytoskeleton in renal mesangial cells.Can J Physiol Pharmacol. 2013 Jan;91(1):1-7. doi: 10.1139/cjpp-2012-0229. Epub 2013 Jan 1. Can J Physiol Pharmacol. 2013. PMID: 23368511 Review.
Cited by
-
Characterization of proteins regulated by interleukin-4 in 3T3-L1 adipocytes.Springerplus. 2015 Jun 4;4:242. doi: 10.1186/s40064-015-0980-0. eCollection 2015. Springerplus. 2015. PMID: 26110103 Free PMC article.
-
Force spectroscopy measurements show that cortical neurons exposed to excitotoxic agonists stiffen before showing evidence of bleb damage.PLoS One. 2013 Aug 30;8(8):e73499. doi: 10.1371/journal.pone.0073499. eCollection 2013. PLoS One. 2013. PMID: 24023686 Free PMC article.
-
CapZ-lipid membrane interactions: a computer analysis.Theor Biol Med Model. 2006 Aug 16;3:30. doi: 10.1186/1742-4682-3-30. Theor Biol Med Model. 2006. PMID: 16914033 Free PMC article.
-
Embryonic Temperature Influences the Mucosal Responses of Atlantic Salmon Alevins to a Bacterial Challenge.Mar Biotechnol (NY). 2024 Nov 19;27(1):1. doi: 10.1007/s10126-024-10386-w. Mar Biotechnol (NY). 2024. PMID: 39560796 Free PMC article.
-
Supervillin reorganizes the actin cytoskeleton and increases invadopodial efficiency.Mol Biol Cell. 2009 Feb;20(3):948-62. doi: 10.1091/mbc.e08-08-0867. Epub 2008 Dec 24. Mol Biol Cell. 2009. PMID: 19109420 Free PMC article.
References
-
- dos Remedios C. G., Chhabra D., Kekic M., Dedova I. V., Tsubakihara M., Berry D. A., Noseworthy N. J. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol. Rev. 2003;83:433–473. - PubMed
-
- Isenberg G., Goldmann W. H. Actin-binding protein–lipid interaction. In: Pryme H., editor. The Cytoskeleton, vol. 1. Greenwich, CT/London: JAI Press Inc.; 1995. pp. 169–204.
-
- Irvine R. F., Schell M. J. Back in the water: the return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2001;2:327–338. - PubMed
-
- Ellson C. D., Andrews S., Stephens L. R., Hawkins P. T. The PX domain: a new phosphoinositide-binding module. J. Cell Sci. 2002;115:1099–1105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

