Oscillatory activity within rat substantia gelatinosa in vitro: a role for chemical and electrical neurotransmission
- PMID: 15528239
- PMCID: PMC1665482
- DOI: 10.1113/jphysiol.2004.076398
Oscillatory activity within rat substantia gelatinosa in vitro: a role for chemical and electrical neurotransmission
Abstract
Although rhythmic behaviour of mammalian spinal ventral horn networks has been extensively studied little is known about oscillogenesis in the spinal dorsal horn. The aims of this in vitro study were to record and determine the underlying mechanisms of potassium-evoked network field oscillations in the substantia gelatinosa of the neonatal rat dorsal horn, a lamina involved in nociceptive processing. Transient pressure ejection of a potassium solution evoked reproducible rhythmic activity in discrete areas of the substantia gelatinosa which lasted for 5-15 s with a single prominent peak in the 4-12 Hz frequency band (7.7 +/- 0.1 Hz, n = 60). Oscillations of similar frequency and amplitude were also observed in isolated dorsal horn quadrants. Application of CNQX (10 microm) reduced peak power amplitude and integrated power area (from 4 to 12 Hz) of the power spectrum, whereas D-AP5 (50 microm) had no effect on the potassium-evoked rhythm. Bicuculline (30 microm) or strychnine (10 microm) reduced the power amplitude and area. On combination of bicuculline (30 microm) and strychnine (10 microm) the reductions in power amplitude and area were not significantly different (P > 0.05) when compared with application of either drug alone. The gap junction blockers carbenoxolone (100 microm) or octanol (1 mM) significantly reduced power amplitude and area. Although TTX (1 microm) or a calcium-free perfusate both caused reductions in the power amplitude and area, potassium-evoked rhythmic activity persisted. However, this persistent rhythm was further reduced on combination of calcium-free perfusate with octanol (1 mM) and was abolished using a cocktail of drugs. Blockade of the potassium delayed rectifier current by tetraethylammonium (5 mM) or the hyperpolarization-activated current (I(h)) by ZD7288 (10 microm) disrupted the synchronization of the potassium-induced oscillation. The frequency of potassium-induced rhythms was unaffected by any of the drugs tested. These novel findings demonstrate that transient pressure ejection of potassium evokes oscillatory activity in the substantia gelatinosa in vitro. This rhythm is partly dependent upon various receptors (AMPA/kainate, GABA(A) and glycine), ion channels (potassium delayed rectifier and I(h)) and gap junctions. Oscillatory behaviour in the substantia gelatinosa could potentially play a role in the processing of nociceptive signals.
Figures
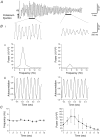
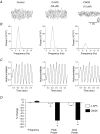




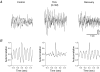
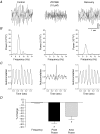
References
-
- Asghar AUR, Al Dawoud M, LeBeau FEN, Buhl EH, King AE. The role of GABAA and glycine receptor-mediated inhibition of theta frequency oscillations in substantia gelatinosa neurones of the rat spinal cord in vitro. J Physiol. 2002a;544.P:78P.
-
- Asghar AUR, LeBeau FE, Buhl EH, King AE. A role for gap junctions in theta oscillations in substantia gelatinosa neurones of the young rat spinal cord in vitro. FENS Abstracts. 2002b;1:273.
-
- Asghar AUR, LeBeau FE, Buhl EH, King AE. Potassium-induced theta oscillations in rat substantia gelatinosa neurones of the spinal cord in vitro. J Physiol. 2002c;539.P:152P.
-
- Baranauskas G, Nistri A. Membrane potential oscillations of neonatal rat spinal motoneurons evoked by electrical stimulation of dorsal root fibres. Eur J Neurosci. 1995;7:2403–2408. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

