Frequency-dependent acceleration of relaxation in mammalian heart: a property not relying on phospholamban and SERCA2a phosphorylation
- PMID: 15528241
- PMCID: PMC1665530
- DOI: 10.1113/jphysiol.2004.075432
Frequency-dependent acceleration of relaxation in mammalian heart: a property not relying on phospholamban and SERCA2a phosphorylation
Abstract
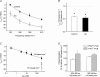
An increase in stimulation frequency causes an acceleration of myocardial relaxation (FDAR). Several mechanisms have been postulated to explain this effect, among which is the Ca(2+)-calmodulin-dependent protein kinase (CaMKII)-dependent phosphorylation of the Thr(17) site of phospholamban (PLN). To gain further insights into the mechanisms of FDAR, we studied the FDAR and the phosphorylation of PLN residues in perfused rat hearts, cat papillary muscles and isolated cat myocytes. This allowed us to sweep over a wide range of frequencies, in species with either positive or negative force-frequency relationships, as well as to explore the FDAR under isometric (or isovolumic) and isotonic conditions. Results were compared with those produced by isoprenaline, an intervention known to accelerate relaxation (IDAR) via PLN phosphorylation. While IDAR occurs tightly associated with a significant increase in the phosphorylation of Ser(16) and Thr(17) of PLN, FDAR occurs without significant changes in the phosphorylation of PLN residues in the intact heart and cat papillary muscles. Moreover, in intact hearts, FDAR was not associated with any significant change in the CaMKII-dependent phosphorylation of sarcoplasmic/endoplasmic Ca(2+) ATPase (SERCA2a), and was not affected by the presence of the CaMKII inhibitor, KN-93. In isolated myocytes, FDAR occurred associated with an increase in Thr(17) phosphorylation. However, for a similar relaxant effect produced by isoprenaline, the phosphorylation of PLN (Ser(16) and Thr(17)) was significantly higher in the presence of the beta-agonist. Moreover, the time course of Thr(17) phosphorylation was significantly delayed with respect to the onset of FDAR. In contrast, the time course of Ser(16) phosphorylation, the first residue that becomes phosphorylated with isoprenaline, was temporally associated with IDAR. Furthermore, KN-93 significantly decreased the phosphorylation of Thr(17) that was evoked by increasing the stimulation frequency, but failed to affect FDAR. Taken together, the results provide direct evidence indicating that CaMKII phosphorylation pathways are not involved in FDAR and that FDAR and IDAR do not share a common underlying mechanism. More likely, a CaMKII-independent mechanism could be involved, whereby increasing stimulation frequency would disrupt the SERCA2a-PLN interaction, leading to an increase in SR Ca(2+) uptake and myocardial relaxation.
Figures
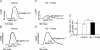
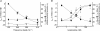

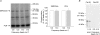

Similar articles
-
Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions.Cardiovasc Res. 2005 Dec 1;68(3):366-75. doi: 10.1016/j.cardiores.2005.08.010. Epub 2005 Oct 13. Cardiovasc Res. 2005. PMID: 16226237 Review.
-
Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban.J Mol Cell Cardiol. 2002 Aug;34(8):975-84. doi: 10.1006/jmcc.2002.2034. J Mol Cell Cardiol. 2002. PMID: 12234767
-
CaMKII effects on inotropic but not lusitropic force frequency responses require phospholamban.J Mol Cell Cardiol. 2012 Sep;53(3):429-36. doi: 10.1016/j.yjmcc.2012.06.019. Epub 2012 Jul 11. J Mol Cell Cardiol. 2012. PMID: 22796260 Free PMC article.
-
Temporal dissociation of frequency-dependent acceleration of relaxation and protein phosphorylation by CaMKII.J Mol Cell Cardiol. 2007 Mar;42(3):590-9. doi: 10.1016/j.yjmcc.2006.12.007. Epub 2006 Dec 21. J Mol Cell Cardiol. 2007. PMID: 17239900 Free PMC article.
-
Phospholamban and cardiac function: a comparative perspective in vertebrates.Acta Physiol (Oxf). 2012 May;205(1):9-25. doi: 10.1111/j.1748-1716.2012.02389.x. Acta Physiol (Oxf). 2012. PMID: 22463608 Review.
Cited by
-
Synergy between CaMKII substrates and β-adrenergic signaling in regulation of cardiac myocyte Ca(2+) handling.Biophys J. 2010 Oct 6;99(7):2038-47. doi: 10.1016/j.bpj.2010.08.016. Biophys J. 2010. PMID: 20923637 Free PMC article.
-
Changes in contractile protein expression are linked to ventricular stiffness in infants with pulmonary hypertension or right ventricular hypertrophy due to congenital heart disease.Open Heart. 2018 Jan 3;5(1):e000716. doi: 10.1136/openhrt-2017-000716. eCollection 2018. Open Heart. 2018. PMID: 29344379 Free PMC article.
-
Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban.J Mol Cell Cardiol. 2007 Sep;43(3):354-61. doi: 10.1016/j.yjmcc.2007.06.013. Epub 2007 Jul 10. J Mol Cell Cardiol. 2007. PMID: 17689560 Free PMC article.
-
Measurement and modeling of Ca2+ waves in isolated rabbit ventricular cardiomyocytes.Biophys J. 2007 Oct 1;93(7):2581-95. doi: 10.1529/biophysj.106.102293. Epub 2007 Jun 1. Biophys J. 2007. PMID: 17545234 Free PMC article.
-
SERCA is critical to control the Bowditch effect in the heart.Sci Rep. 2018 Aug 20;8(1):12447. doi: 10.1038/s41598-018-30638-9. Sci Rep. 2018. PMID: 30127403 Free PMC article.
References
-
- Asahi M, McKenna E, Kurzydlowski K, Tada M, MacLennan DH. Physical interactions between phospholamban and sarco(endo)plasmic reticulum Ca2+-ATPases are dissociated by elevated Ca2+, but not by phospholamban phosphorylation, vanadate, or thapsigargin, and are enhanced by ATP. J Biol Chem. 2000;275:15034–15038. 10.1074/jbc.275.20.15034. - DOI - PubMed
-
- Bassani RA, Mattiazzi A, Bers DM. CaMKII is responsible for activity-dependent acceleration of relaxation in rat ventricular myocytes. Am J Physiol. 1995;268:H703–H712. - PubMed
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd edn. The Netherlands: Kluwer Academic Publishers; 2000.
-
- Bluhm WF, Kranias EG, Dillmann WH, Meyer M. Phospholamban: a major determinant of the cardiac force-frequency relationship. Am J Physiol. 2000;278:H249–H255. - PubMed
-
- Champion HC, Georgakopoulos D, Haldar S, Wang L, Wang Y, Kass DA. Robust adenoviral and adeno-associated viral gene transfer to the in vivo murine heart: application to study of phospholamban physiology. Circulation. 2003;108:2790–2797. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

