Voltage-controlled Ca2+ release and entry flux in isolated adult muscle fibres of the mouse
- PMID: 15528246
- PMCID: PMC1665514
- DOI: 10.1113/jphysiol.2004.073882
Voltage-controlled Ca2+ release and entry flux in isolated adult muscle fibres of the mouse
Abstract
The voltage-activated fluxes of Ca(2+) from the sarcoplasmic reticulum (SR) and from the extracellular space were studied in skeletal muscle fibres of adult mice. Single fibres of the interosseus muscle were enzymatically isolated and voltage clamped using a two-electrode technique. The fibres were perfused from the current-passing micropipette with a solution containing 15 mm EGTA and 0.2 mm of either fura-2 or the faster, lower affinity indicator fura-FF. Electrical recordings in parallel with the fluorescence measurements allowed the estimation of intramembrane gating charge movements and transmembrane Ca(2+) inward current exhibiting half-maximal activation at -7.60 +/- 1.29 and 3.0 +/- 1.44 mV, respectively. The rate of Ca(2+) release from the SR was calculated after fitting the relaxation phases of fluorescence ratio signals with a kinetic model to quantify overall Ca(2+) removal. Results obtained with the two indicators were similar. Ca(2+) release was 2-3 orders of magnitude larger than the flux carried by the L-type Ca(2+) current. At maximal depolarization (+50 mV), release flux peaked at about 3 ms after the onset of the voltage pulse and then decayed in two distinct phases. The slower phase, most likely resulting from SR depletion, indicated a decrease in lumenal Ca(2+) content by about 80% within 100 ms. Unlike in frog fibres, the kinetics of the rapid phase of decay showed no dependence on the filling state of the SR and the results provide little evidence for a substantial increase of SR permeability on depletion. The approach described here promises insight into excitation-contraction coupling in future studies of genetically altered mice.
Figures
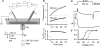




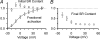



Similar articles
-
Altered inactivation of Ca2+ current and Ca2+ release in mouse muscle fibers deficient in the DHP receptor gamma1 subunit.J Gen Physiol. 2004 Nov;124(5):605-18. doi: 10.1085/jgp.200409168. J Gen Physiol. 2004. PMID: 15504904 Free PMC article.
-
Modification of excitation-contraction coupling by 4-chloro-m-cresol in voltage-clamped cut muscle fibres of the frog (R. pipiens).J Physiol. 1999 Feb 15;515 ( Pt 1)(Pt 1):221-31. doi: 10.1111/j.1469-7793.1999.221ad.x. J Physiol. 1999. PMID: 9925891 Free PMC article.
-
Intramembrane charge movement and sarcoplasmic calcium release in enzymatically isolated mammalian skeletal muscle fibres.J Physiol. 1997 Dec 1;505 ( Pt 2)(Pt 2):371-84. doi: 10.1111/j.1469-7793.1997.371bb.x. J Physiol. 1997. PMID: 9423180 Free PMC article.
-
A study of the mechanisms of excitation-contraction coupling in frog skeletal muscle based on measurements of [Ca2+] transients inside the sarcoplasmic reticulum.J Muscle Res Cell Motil. 2018 Apr;39(1-2):41-60. doi: 10.1007/s10974-018-9497-9. Epub 2018 Aug 24. J Muscle Res Cell Motil. 2018. PMID: 30143958 Review.
-
Voltage clamp methods for the study of membrane currents and SR Ca(2+) release in adult skeletal muscle fibres.Prog Biophys Mol Biol. 2012 Apr;108(3):98-118. doi: 10.1016/j.pbiomolbio.2012.01.001. Epub 2012 Jan 26. Prog Biophys Mol Biol. 2012. PMID: 22306655 Free PMC article. Review.
Cited by
-
Evolution and modulation of intracellular calcium release during long-lasting, depleting depolarization in mouse muscle.J Physiol. 2008 Oct 1;586(19):4609-29. doi: 10.1113/jphysiol.2008.157990. Epub 2008 Aug 7. J Physiol. 2008. PMID: 18687715 Free PMC article.
-
Major contribution of sarcoplasmic reticulum Ca(2+) depletion during long-lasting activation of skeletal muscle.J Gen Physiol. 2013 May;141(5):557-65. doi: 10.1085/jgp.201310957. J Gen Physiol. 2013. PMID: 23630339 Free PMC article.
-
ECC meets CEU-New focus on the backdoor for calcium ions in skeletal muscle cells.J Gen Physiol. 2020 Oct 5;152(10):e202012679. doi: 10.1085/jgp.202012679. J Gen Physiol. 2020. PMID: 32851409 Free PMC article.
-
Measurement of intracellular ion activity in skeletal muscle fibers: Four microelectrodes or no deal.J Gen Physiol. 2019 Oct 7;151(10):1160-1162. doi: 10.1085/jgp.201912425. Epub 2019 Aug 30. J Gen Physiol. 2019. PMID: 31471451 Free PMC article.
-
Synthetic localized calcium transients directly probe signalling mechanisms in skeletal muscle.J Physiol. 2012 Mar 15;590(6):1389-411. doi: 10.1113/jphysiol.2011.225854. Epub 2012 Feb 6. J Physiol. 2012. PMID: 22310315 Free PMC article.
References
-
- Adams BA, Beam KG. Muscular dysgenesis in mice: a model system for studying excitation-contraction coupling. FASEB J. 1990;4:2809–2816. - PubMed
-
- Bakker AJ, Head SI, Stephenson DG. Time course of calcium transients derived from Fura-2 fluorescence measurements in single fast twitch fibres of adult mice and rat myotubes developing in primary culture. Cell Calcium. 1997;21:359–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

