Localization and function of the Kv3.1b subunit in the rat medulla oblongata: focus on the nucleus tractus solitarii
- PMID: 15528247
- PMCID: PMC1665536
- DOI: 10.1113/jphysiol.2004.073338
Localization and function of the Kv3.1b subunit in the rat medulla oblongata: focus on the nucleus tractus solitarii
Abstract
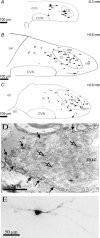
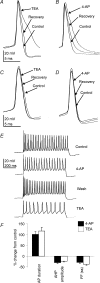
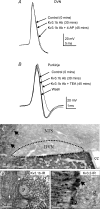
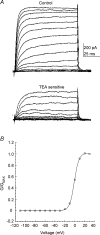
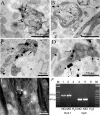
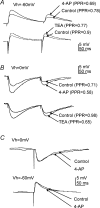
The voltage-gated potassium channel subunit Kv3.1 confers fast firing characteristics to neurones. Kv3.1b subunit immunoreactivity (Kv3.1b-IR) was widespread throughout the medulla oblongata, with labelled neurones in the gracile, cuneate and spinal trigeminal nuclei. In the nucleus of the solitary tract (NTS), Kv3.1b-IR neurones were predominantly located close to the tractus solitarius (TS) and could be GABAergic or glutamatergic. Ultrastructurally, Kv3.1b-IR was detected in NTS terminals, some of which were vagal afferents. Whole-cell current-clamp recordings from neurones near the TS revealed electrophysiological characteristics consistent with the presence of Kv3.1b subunits: short duration action potentials (4.2 +/- 1.4 ms) and high firing frequencies (68.9 +/- 5.3 Hz), both sensitive to application of TEA (0.5 mm) and 4-aminopyridine (4-AP; 30 mum). Intracellular dialysis of an anti-Kv3.1b antibody mimicked and occluded the effects of TEA and 4-AP in NTS and dorsal column nuclei neurones, but not in dorsal vagal nucleus or cerebellar Purkinje cells (which express other Kv3 subunits, but not Kv3.1b). Voltage-clamp recordings from outside-out patches from NTS neurones revealed an outward K(+) current with the basic characteristics of that carried by Kv3 channels. In NTS neurones, electrical stimulation of the TS evoked EPSPs and IPSPs, and TEA and 4-AP increased the average amplitude and decreased the paired pulse ratio, consistent with a presynaptic site of action. Synaptic inputs evoked by stimulation of a region lacking Kv3.1b-IR neurones were not affected, correlating the presence of Kv3.1b in the TS with the pharmacological effects.
Figures



Similar articles
-
Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance.Physiol Rev. 2017 Oct 1;97(4):1431-1468. doi: 10.1152/physrev.00002.2017. Physiol Rev. 2017. PMID: 28904001 Free PMC article. Review.
-
Immunohistochemical localisation of the voltage gated potassium ion channel subunit Kv3.3 in the rat medulla oblongata and thoracic spinal cord.Brain Res. 2006 Jan 27;1070(1):101-15. doi: 10.1016/j.brainres.2005.10.102. Epub 2006 Jan 5. Brain Res. 2006. PMID: 16403474
-
Voltage-gated potassium currents within the dorsal vagal nucleus: inhibition by BDS toxin.Brain Res. 2008 Jan 16;1189:51-7. doi: 10.1016/j.brainres.2007.10.090. Epub 2007 Nov 9. Brain Res. 2008. PMID: 18048010
-
Properties of interneurones in the intermediolateral cell column of the rat spinal cord: role of the potassium channel subunit Kv3.1.Neuroscience. 2001;106(2):433-46. doi: 10.1016/s0306-4522(01)00277-9. Neuroscience. 2001. PMID: 11566512
-
Going native: voltage-gated potassium channels controlling neuronal excitability.J Physiol. 2010 Sep 1;588(Pt 17):3187-200. doi: 10.1113/jphysiol.2010.191973. Epub 2010 Jun 2. J Physiol. 2010. PMID: 20519310 Free PMC article. Review.
Cited by
-
Kv3.1b and Kv3.3 channel subunit expression in murine spinal dorsal horn GABAergic interneurones.J Chem Neuroanat. 2011 Sep;42(1):30-8. doi: 10.1016/j.jchemneu.2011.02.003. Epub 2011 Apr 1. J Chem Neuroanat. 2011. PMID: 21440618 Free PMC article.
-
Somatic membrane potential and Kv1 channels control spike repolarization in cortical axon collaterals and presynaptic boutons.J Neurosci. 2011 Oct 26;31(43):15490-8. doi: 10.1523/JNEUROSCI.2752-11.2011. J Neurosci. 2011. PMID: 22031895 Free PMC article.
-
Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance.Physiol Rev. 2017 Oct 1;97(4):1431-1468. doi: 10.1152/physrev.00002.2017. Physiol Rev. 2017. PMID: 28904001 Free PMC article. Review.
-
Localization and targeting of voltage-dependent ion channels in mammalian central neurons.Physiol Rev. 2008 Oct;88(4):1407-47. doi: 10.1152/physrev.00002.2008. Physiol Rev. 2008. PMID: 18923186 Free PMC article. Review.
References
-
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels Kv1.5 and Kv2.1 in hypoxic pulmonary vasoconstriction, and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. - PMC - PubMed
-
- Atkinson L, Deuchars J. Distribution of Kv3.1b immunoreactivity in the dorsomedial medulla of the rat. Proc Physiol Soc. 2000;526.P:171.
-
- Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci. 2003;6:258–266. - PubMed
-
- Barraco R, El-Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull. 1992;29:703. - PubMed
-
- Berger AJ. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Res. 1977;135:231–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

