Neuronal mechanisms mediating the variability of somatosensory evoked potentials during sleep oscillations in cats
- PMID: 15528249
- PMCID: PMC1665518
- DOI: 10.1113/jphysiol.2004.071381
Neuronal mechanisms mediating the variability of somatosensory evoked potentials during sleep oscillations in cats
Abstract
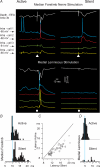
The slow oscillation (SO) generated within the corticothalamic system is composed of active and silent states. The studies of response variability during active versus silent network states within thalamocortical system of human and animals provided inconsistent results. To investigate this inconsistency, we used electrophysiological recordings from the main structures of the somatosensory system in anaesthetized cats. Stimulation of the median nerve (MN) elicited cortical responses during all phases of SO. Cortical responses to stimulation of the medial lemniscus (ML) were virtually absent during silent periods. At the ventral-posterior lateral (VPL) level, ML stimuli elicited either EPSPs in isolation or EPSPs crowned by spikes, as a function of membrane potential. Response to MN stimuli elicited compound synaptic responses and spiked at any physiological level of membrane potential. The responses of dorsal column nuclei neurones to MN stimuli were of similar latency, but the latencies of antidromic responses to ML stimuli were variable. Thus, the variable conductance velocity of ascending prethalamic axons was the most likely cause of the barrages of synaptic events in VPL neurones mediating their firing at different level of the membrane potential. We conclude that the preserved ability of the somatosensory system to transmit the peripheral stimuli to the cerebral cortex during all the phases of sleep slow oscillation is based on the functional properties of the medial lemniscus and on the intrinsic properties of the thalamocortical cells. However the reduced firing ability of the cortical neurones during the silent state may contribute to impair sensory processing during sleep.
Figures





References
-
- Alloway KD, Wallace MB, Johnson MJ. Cross-correlation analysis of cuneothalamic interactions in the rat somatosensory system: influence of receptive field topography and comparisons with thalamocortical interactions. J Neurophysiol. 1994;72:1949–1972. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

