Frequency and spatial distribution of environmental Campylobacter spp
- PMID: 15528512
- PMCID: PMC525266
- DOI: 10.1128/AEM.70.11.6501-6511.2004
Frequency and spatial distribution of environmental Campylobacter spp
Abstract
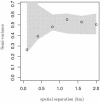
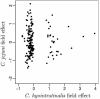
Humans are exposed to Campylobacter spp. in a range of sources via both food and environmental pathways. For this study, we explored the frequency and distribution of thermophilic Campylobacter spp. in a 10- by 10-km square rural area of Cheshire, United Kingdom. The area contains approximately 70, mainly dairy, farms and is used extensively for outdoor recreational activities. Campylobacter spp. were isolated from a range of environmental samples by use of a systematic sampling grid. Livestock (mainly cattle) and wildlife feces and environmental water and soil samples were cultured, and isolates were presumptively identified by standard techniques. These isolates were further characterized by PCR. Campylobacter jejuni was the most prevalent species in all animal samples, ranging from 11% in samples from nonavian wildlife to 36% in cattle feces, and was isolated from 15% of water samples. Campylobacter coli was commonly found in water (17%) and sheep (21%) samples, but rarely in other samples. Campylobacter lari was recovered from all sample types, with the exception of sheep feces, and was found in moderate numbers in birds (7%) and water (5%). Campylobacter hyointestinalis was only recovered from cattle (7%) and birds (1%). The spatial distribution and determinants of C. jejuni in cattle feces were examined by the use of model-based spatial statistics. The distribution was consistent with very localized within-farm or within-field transmission and showed little evidence of any larger-scale spatial dependence. We concluded that there is a potentially high risk of human exposure to Campylobacter spp., particularly C. jejuni, in the environment of our study area. The prevalence and likely risk posed by C. jejuni-positive cattle feces in the environment diminished as the fecal material aged. After we took into account the age of the fecal material, the absence or presence of rain, and the presence of bird feces, there was evidence of significant variation in the prevalence of C. jejuni-positive cattle feces between grazing fields but no evidence of spatial clustering beyond this resolution. The spatial pattern of C. jejuni is therefore consistent with that for an organism that is ubiquitous in areas contaminated with cattle feces, with a short-scale variation in infection intensity that cannot be explained solely by variations in the age of the fecal material. The observed pattern is not consistent with large-scale transmission attributable to watercourses, wildlife territories, or other geographical features that transcend field and farm boundaries.
Figures





References
-
- Barrett, N. J. 1986. Communicable disease associated with milk and dairy-products in England and Wales—1983-1984. J. Infect. 12:265-272. - PubMed
-
- Besag, J., P. Green, D. Higdon, and K. Mengersen. 1995. Bayesian computation and stochastic systems. Statist. Sci. 10:3-66.
-
- CDSC. 2000. Sentinel surveillance of Campylobacter in England and Wales. Commun. Dis. Rep. Wkly. 10:169, 172. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

