Engineering of chimeric class II polyhydroxyalkanoate synthases
- PMID: 15528546
- PMCID: PMC525123
- DOI: 10.1128/AEM.70.11.6789-6799.2004
Engineering of chimeric class II polyhydroxyalkanoate synthases
Abstract
PHA synthase is a key enzyme involved in the biosynthesis of polyhydroxyalkanoates (PHAs). Using a combinatorial genetic strategy to create unique chimeric class II PHA synthases, we have obtained a number of novel chimeras which display improved catalytic properties. To engineer the chimeric PHA synthases, we constructed a synthetic phaC gene from Pseudomonas oleovorans (phaC1Po) that was devoid of an internal 540-bp fragment. Randomly amplified PCR products (created with primers based on conserved phaC sequences flanking the deleted internal fragment) were generated using genomic DNA isolated from soil and were substituted for the 540-bp internal region. The chimeric genes were expressed in a PHA-negative strain of Ralstonia eutropha, PHB(-)4 (DSM 541). Out of 1,478 recombinant clones screened for PHA production, we obtained five different chimeric phaC1Po genes that produced more PHA than the native phaC1Po. Chimeras S1-71, S4-8, S5-58, S3-69, and S3-44 exhibited 1.3-, 1.4-, 2.0-, 2.1-, and 3.0-fold-increased levels of in vivo activity, respectively. All of the mutants mediated the synthesis of PHAs with a slightly increased molar fraction of 3-hydroxyoctanoate; however, the weight-average molecular weights (Mw) of the PHAs in all cases remained almost the same. Based upon DNA sequence analyses, the various phaC fragments appear to have originated from Pseudomonas fluorescens and Pseudomonas aureofaciens. The amino acid sequence analyses showed that the chimeric proteins had 17 to 20 amino acid differences from the wild-type phaC1Po, and these differences were clustered in the same positions in the five chimeric clones. A threading model of PhaC1Po, developed based on homology of the enzyme to the Burkholderia glumae lipase, suggested that the amino acid substitutions found in the active chimeras were located mostly on the protein model surface. Thus, our combinatorial genetic engineering strategy proved to be broadly useful for improving the catalytic activities of PHA synthase enzymes.
Figures



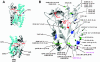
Similar articles
-
Incremental truncation of PHA synthases results in altered product specificity.Enzyme Microb Technol. 2012 May 10;50(6-7):293-7. doi: 10.1016/j.enzmictec.2012.02.003. Epub 2012 Feb 25. Enzyme Microb Technol. 2012. PMID: 22500895
-
Chimeric enzyme composed of polyhydroxyalkanoate (PHA) synthases from Ralstonia eutropha and Aeromonas caviae enhances production of PHAs in recombinant Escherichia coli.Biomacromolecules. 2009 Apr 13;10(4):682-5. doi: 10.1021/bm801386j. Biomacromolecules. 2009. PMID: 19226108
-
Molecular characterization of the poly(3-hydroxybutyrate) (PHB) synthase from Ralstonia eutropha: in vitro evolution, site-specific mutagenesis and development of a PHB synthase protein model.Biochim Biophys Acta. 2002 Jan 31;1594(1):178-90. doi: 10.1016/s0167-4838(01)00299-0. Biochim Biophys Acta. 2002. PMID: 11825620
-
PHA synthase (PhaC): interpreting the functions of bioplastic-producing enzyme from a structural perspective.Appl Microbiol Biotechnol. 2019 Feb;103(3):1131-1141. doi: 10.1007/s00253-018-9538-8. Epub 2018 Dec 3. Appl Microbiol Biotechnol. 2019. PMID: 30511262 Review.
-
Polyhydroyxalkanoate synthase fusions as a strategy for oriented enzyme immobilisation.Molecules. 2014 Jun 24;19(6):8629-43. doi: 10.3390/molecules19068629. Molecules. 2014. PMID: 24962396 Free PMC article. Review.
Cited by
-
Contribution of the distal pocket residue to the acyl-chain-length specificity of (R)-specific enoyl-coenzyme A hydratases from Pseudomonas spp.Appl Environ Microbiol. 2015 Dec;81(23):8076-83. doi: 10.1128/AEM.02412-15. Epub 2015 Sep 18. Appl Environ Microbiol. 2015. PMID: 26386053 Free PMC article.
-
Biotechnological Approaches for Enhancing Polyhydroxyalkanoates (PHAs) Production: Current and Future Perspectives.Curr Microbiol. 2023 Sep 20;80(11):345. doi: 10.1007/s00284-023-03452-4. Curr Microbiol. 2023. PMID: 37731015 Review.
References
-
- Clemente, T., D. Shah, M. Tran, D. Stark, S. Padgette, D. Dennis, K. Brückener, A. Steinbüchel, and T. A. Mitsky. 2000. Sequence of PHA synthase gene from two strains of Rhodospirillum rubrum and in vivo substrate specificity of four PHA synthases across two heterologous expression systems. Appl. Microbiol. Biotechnol. 53:420-429. - PubMed
-
- Green, P. R., J. Kemper, L. Schechtman, L. Guo, M. Satkowski, S. Fiedler, A. Steinbüchel, and B. H. A. Rehm. 2002. Formation of short chain length/medium chain length polyhydroxyalkanoate copolymers by fatty acid β-oxidation inhibited Ralstonia eutropha. Biomacromolecules 3:208-213. - PubMed
-
- Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, and S. T. Williams. 1993. Bergey's manual of determinative bacteriology, 9th ed. Williams & Wilkins, Baltimore, Md.
-
- Jia, Y., T. J. Kappock, T. Frick, A. J. Sinskey, and J. Stubbe. 2000. Lipases provide a new mechanistic model for polyhydroxybutyrate (PHB) synthases: characterization of the functional residues in Chromatium vinosum PHB synthase. Biochemistry 39:3927-3936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

