Intracellular proliferation of Legionella pneumophila in Hartmannella vermiformis in aquatic biofilms grown on plasticized polyvinyl chloride
- PMID: 15528550
- PMCID: PMC525122
- DOI: 10.1128/AEM.70.11.6826-6833.2004
Intracellular proliferation of Legionella pneumophila in Hartmannella vermiformis in aquatic biofilms grown on plasticized polyvinyl chloride
Abstract
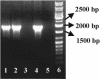
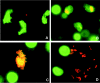
The need for protozoa for the proliferation of Legionella pneumophila in aquatic habitats is still not fully understood and is even questioned by some investigators. This study shows the in vivo growth of L. pneumophila in protozoa in aquatic biofilms developing at high concentrations on plasticized polyvinyl chloride in a batch system with autoclaved tap water. The inoculum, a mixed microbial community including indigenous L. pneumophila originating from a tap water system, was added in an unfiltered as well as filtered (cellulose nitrate, 3.0-microm pore size) state. Both the attached and suspended biomasses were examined for their total amounts of ATP, for culturable L. pneumophila, and for their concentrations of protozoa. L. pneumophila grew to high numbers (6.3 log CFU/cm2) only in flasks with an unfiltered inoculum. Filtration obviously removed the growth-supporting factor, but it did not affect biofilm formation, as determined by measuring ATP. Cultivation, direct counting, and 18S ribosomal DNA-targeted PCR with subsequent sequencing revealed the presence of Hartmannella vermiformis in all flasks in which L. pneumophila multiplied and also when cycloheximide had been added. Fluorescent in situ hybridization clearly demonstrated the intracellular growth of L. pneumophila in trophozoites of H. vermiformis, with 25.9% +/- 10.5% of the trophozoites containing L. pneumophila on day 10 and >90% containing L. pneumophila on day 14. Calculations confirmed that intracellular growth was most likely the only way for L. pneumophila to proliferate within the biofilm. Higher biofilm concentrations, measured as amounts of ATP, gave higher L. pneumophila concentrations, and therefore the growth of L. pneumophila within engineered water systems can be limited by controlling biofilm formation.
Figures



References
-
- Amaral Zettler, L. A., T. A. Nerad, C. J. O'Kelly, M. T. Peglar, P. M. Gillevet, J. D. Silberman, and M. L. Sogin. 2000. A molecular reassessment of the leptomyxid amoebae. Protist 151:275-282. - PubMed
-
- Arndt, H. 1993. A critical review of the importance of rhizopods (naked and testate amoebae) and actinopods (heliozoa) in lake plankton. Mar. Microb. Food Webs 7:3-29.
-
- Baldock, B. M., J. H. Baker, and M. A. Sleigh. 1980. Laboratory growth rates of six species of freshwater gymnamoebia. Oecologia 47:156-159. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

