Degradation of a nonylphenol single isomer by Sphingomonas sp. strain TTNP3 leads to a hydroxylation-induced migration product
- PMID: 15528560
- PMCID: PMC525215
- DOI: 10.1128/AEM.70.11.6897-6900.2004
Degradation of a nonylphenol single isomer by Sphingomonas sp. strain TTNP3 leads to a hydroxylation-induced migration product
Abstract
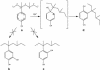
Sphingomonas sp. strain TTNP3 degrades 4(3',5'-dimethyl-3'-heptyl)-phenol and unidentified metabolites that were described previously. The chromatographic analyses of the synthesized reference compound and the metabolites led to their identification as 2(3',5'-dimethyl-3'-heptyl)-1,4-benzenediol. This finding indicates that the nonylphenol metabolism of this bacterium involves unconventional degradation pathways where an NIH shift mechanism occurs.
Figures

Similar articles
-
Contribution to the Detection and Identification of Oxidation Metabolites of Nonylphenol in Sphingomonas sp. strain TTNP3.Biodegradation. 2007 Apr;18(2):233-45. doi: 10.1007/s10532-006-9058-6. Epub 2006 Jul 5. Biodegradation. 2007. PMID: 16821104
-
Microbial degradation of a single branched isomer of nonylphenol by Sphingomonas TTNP3.Water Sci Technol. 2004;50(5):189-94. Water Sci Technol. 2004. PMID: 15497847
-
The degradation of alpha-quaternary nonylphenol isomers by Sphingomonas sp. strain TTNP3 involves a type II ipso-substitution mechanism.Appl Microbiol Biotechnol. 2006 Mar;70(1):114-22. doi: 10.1007/s00253-005-0080-0. Epub 2005 Aug 10. Appl Microbiol Biotechnol. 2006. PMID: 16091931
-
The degradation of alkylphenols by Sphingomonas sp. strain TTNP3 - a review on seven years of research.N Biotechnol. 2012 Nov 15;30(1):88-95. doi: 10.1016/j.nbt.2012.07.008. Epub 2012 Jul 27. N Biotechnol. 2012. PMID: 22842087 Review.
-
Microbial degradation of nonylphenol and other alkylphenols--our evolving view.Appl Microbiol Biotechnol. 2006 Sep;72(2):223-43. doi: 10.1007/s00253-006-0476-5. Epub 2006 Jul 7. Appl Microbiol Biotechnol. 2006. PMID: 16826376 Review.
Cited by
-
Purification and characterization of hydroquinone dioxygenase from Sphingomonas sp. strain TTNP3.AMB Express. 2011 May 27;1(1):8. doi: 10.1186/2191-0855-1-8. AMB Express. 2011. PMID: 21906340 Free PMC article.
-
Role of P450 monooxygenases in the degradation of the endocrine-disrupting chemical nonylphenol by the white rot fungus Phanerochaete chrysosporium.Appl Environ Microbiol. 2009 Sep;75(17):5570-80. doi: 10.1128/AEM.02942-08. Epub 2009 Jun 19. Appl Environ Microbiol. 2009. PMID: 19542331 Free PMC article.
-
Biodegradation of 7-Hydroxycoumarin in Pseudomonas mandelii 7HK4 via ipso-Hydroxylation of 3-(2,4-Dihydroxyphenyl)-propionic Acid.Molecules. 2018 Oct 12;23(10):2613. doi: 10.3390/molecules23102613. Molecules. 2018. PMID: 30321993 Free PMC article.
-
Occurrence and biodegradation of nonylphenol in the environment.Int J Mol Sci. 2012;13(1):491-505. doi: 10.3390/ijms13010491. Epub 2012 Jan 4. Int J Mol Sci. 2012. PMID: 22312266 Free PMC article. Review.
-
Comparative Analysis of the Genetic Basis of Branched Nonylphenol Degradation by Sphingobium amiense DSM 16289T and Sphingobium cloacae JCM 10874T.Microbes Environ. 2018 Dec 28;33(4):450-454. doi: 10.1264/jsme2.ME18077. Epub 2018 Dec 5. Microbes Environ. 2018. PMID: 30518740 Free PMC article.
References
-
- Corti, A., S. Frassinetti, G. Vallini, S. D'Antone, C. Fichi, and R. Solaro. 1995. Biotransformation of nonionic surfactants. I. Biotransformation of 4-(1-nonyl)phenol by Candida maltosa isolate. Environ. Pollut. 90:83-87. - PubMed
-
- Corvini, P. F. X., R. Vinken, G. Hommes, B. Schmidt, and M. Dohmann. 2004. Degradation of the radioactive and non-labelled branched 4(3′,5′-dimethyl-3′-heptyl)-phenol nonylphenol isomer by Sphingomonas TTNP3. Biodegradation 15:9-18. - PubMed
-
- Corvini P. F. X., R. Vinken, G. Hommes, M. Mundt, R. Meesters, H. F. Schröder, J. Hollender, and B. Schmidt. 2004. Microbial degradation of a single branched isomer of nonylphenol by Sphingomonas sp. strain TTNP3. Water Sci. Technol. 50(5):195-202. - PubMed
-
- Di Corcia, A., A. Costantino, C. Crescenzi, E. Marinoni, and R. Samperi. 1998. Characterization of recalcitrant intermediates of the branched alkyl side chain of nonylphenol ethoxylate surfactants. Environ. Sci. Technol. 32:2401-2409.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

