Degradation of a nonylphenol single isomer by Sphingomonas sp. strain TTNP3 leads to a hydroxylation-induced migration product
- PMID: 15528560
- PMCID: PMC525215
- DOI: 10.1128/AEM.70.11.6897-6900.2004
Degradation of a nonylphenol single isomer by Sphingomonas sp. strain TTNP3 leads to a hydroxylation-induced migration product
Abstract
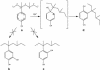
Sphingomonas sp. strain TTNP3 degrades 4(3',5'-dimethyl-3'-heptyl)-phenol and unidentified metabolites that were described previously. The chromatographic analyses of the synthesized reference compound and the metabolites led to their identification as 2(3',5'-dimethyl-3'-heptyl)-1,4-benzenediol. This finding indicates that the nonylphenol metabolism of this bacterium involves unconventional degradation pathways where an NIH shift mechanism occurs.
Figures

References
-
- Corti, A., S. Frassinetti, G. Vallini, S. D'Antone, C. Fichi, and R. Solaro. 1995. Biotransformation of nonionic surfactants. I. Biotransformation of 4-(1-nonyl)phenol by Candida maltosa isolate. Environ. Pollut. 90:83-87. - PubMed
-
- Corvini, P. F. X., R. Vinken, G. Hommes, B. Schmidt, and M. Dohmann. 2004. Degradation of the radioactive and non-labelled branched 4(3′,5′-dimethyl-3′-heptyl)-phenol nonylphenol isomer by Sphingomonas TTNP3. Biodegradation 15:9-18. - PubMed
-
- Corvini P. F. X., R. Vinken, G. Hommes, M. Mundt, R. Meesters, H. F. Schröder, J. Hollender, and B. Schmidt. 2004. Microbial degradation of a single branched isomer of nonylphenol by Sphingomonas sp. strain TTNP3. Water Sci. Technol. 50(5):195-202. - PubMed
-
- Di Corcia, A., A. Costantino, C. Crescenzi, E. Marinoni, and R. Samperi. 1998. Characterization of recalcitrant intermediates of the branched alkyl side chain of nonylphenol ethoxylate surfactants. Environ. Sci. Technol. 32:2401-2409.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

