New multiple antigenic peptide-based enzyme immunoassay for detection of simian immunodeficiency virus infection in nonhuman primates and humans
- PMID: 15528710
- PMCID: PMC525168
- DOI: 10.1128/JCM.42.11.5161-5169.2004
New multiple antigenic peptide-based enzyme immunoassay for detection of simian immunodeficiency virus infection in nonhuman primates and humans
Abstract
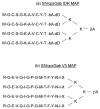
Infections with human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2, respectively) are zoonotic infections. In Africa, the potential exists for additional cross-species transmissions from at least 33 different species of simian immunodeficiency virus (SIV)-infected nonhuman primates (NHPs) through hunting and butchering of these animals for food. Here we describe a highly sensitive and specific enzyme immunoassay (EIA) with chemically modified, multiple antigenic peptides (MAPs) developed for the detection and discrimination of antibodies to SIV genetic lineages. The SIV EIA was developed by using a comprehensive array of MAPs covering two envelope gene regions from all of the SIV lineages for which env sequences were available. Assay sensitivity was evaluated by using 63 plasma or serum samples obtained from primates naturally or experimentally infected with SIVs from 10 genetic lineages. Assay specificity was determined by using 97 known SIV-negative plasma specimens from these same species. Also used in the evaluations were 369 human samples: 198 HIV seronegative, 170 HIV-1 and/or HIV-2 seropositive, and 1 from a human SIVsm infection. Overall assay sensitivity and specificity were 100% with both immunodominant region (IDR) and V3 region MAPs. Although SIV env sequences from talapoin monkeys were not available for specific MAP inclusion, 5 (100%) of 5 SIVtal-infected samples were detected through cross-reactivity with other SIV IDR MAPs used in the assay. The one human SIVsm infection was identified. In conclusion, our SIV MAP EIA proved to be highly sensitive and specific for detecting SIV infections in NHPs and humans. As shown with SIV-infected talapoin monkeys, this assay has the potential to detect previously unidentified SIV strains and should be suitable for sentinel surveillance for potential new cross-species transmissions of SIVs to humans.
Figures




References
-
- Apetrei, C., D. L. Robertson, and P. A. Marx. 2004. The history of SIVs and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front. Biosci. 9:225-254. - PubMed
-
- Beer, B. E., E. Bailes, R. Goeken, G. Dapolito, C. Coulibaly, S. G. Norley, R. Kurth, J. P. Gautier, A. Gautier-Hion, D. Vallet, P. M. Sharp, and V. M. Hirsch. 1999. Simian immunodeficiency virus (SIV) from sun-tailed monkeys (Cercopithecus solatus): evidence for host-dependent evolution of SIV within the C. lhoesti superspecies. J. Virol. 73:7734-7744. - PMC - PubMed
-
- Beer, B. E., B. T. Foley, C. L. Kuiken, Z. Tooze, R. M. Goeken, C. R. Brown, J. Hu, M. St. Claire, B. T. Korber, and V. M. Hirsch. 2001. Characterization of novel simian immunodeficiency viruses from red-capped mangabeys from Nigeria (SIVrcmNG409 and -NG411). J. Virol. 75:12014-12027. - PMC - PubMed
-
- Brattegaard, K., D. Soroh, F. Zadi, H. Digbeu, K. M. Vetter, and K. M. De Cock. 1995. Insensitivity of a synthetic peptide-based test (Pepti-LAV 1-2) for the diagnosis of HIV infection in African children. AIDS 9:656-657. - PubMed
-
- Courgnaud, V., B. Abela, X. Pourrut, E. Mpoudi-Ngole, S. Lout, E. Delaporte, and M. Peeters. 2003. Identification of a new simian immunodeficiency virus lineage with a vpu gene present among different Cercopithecus monkeys (C. mona, C. cephus, and C. nictitans) from Cameroon. J. Virol. 77:12523-12534. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

