Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis
- PMID: 15528719
- PMCID: PMC525189
- DOI: 10.1128/JCM.42.11.5229-5237.2004
Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis
Abstract
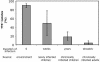
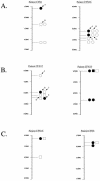
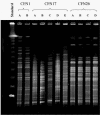
Pseudomonas aeruginosa is a frequent cause of respiratory exacerbations in individuals with cystic fibrosis. An important virulence determinant of this pathogen is its type III protein secretion system. In this study, the type III secretion properties of 435 P. aeruginosa respiratory isolates from 56 chronically infected individuals with cystic fibrosis were investigated. Although it had been previously reported that 75 to 90% of P. aeruginosa isolates from patients with hospital-acquired pneumonia secreted type III proteins, only 12% of isolates from cystic fibrosis patients did so, with nearly all of these isolates secreting ExoS and ExoT but not ExoU. Despite the low overall prevalence of type III protein-secreting isolates, at least one secreting isolate was cultured from one-third of cystic fibrosis patients. Interestingly, the fraction of cystic fibrosis patient isolates capable of secreting type III proteins decreased with duration of infection. Although 90% of isolates from the environment, the presumed reservoir for the majority of P. aeruginosa strains that infect patients with cystic fibrosis, secreted type III proteins, only 49% of isolates from newly infected children, 18% of isolates from chronically infected children, and 4% of isolates from chronically infected adults with cystic fibrosis secreted these proteins. Within individual patients, isolates of clonal origin differed in their secretion phenotypes, indicating that as strains persisted in cystic fibrosis patient airways, their type III protein secretion properties changed. Together, these findings indicate that following infection of cystic fibrosis patient airways, P. aeruginosa strains gradually change from a type III protein secretion-positive phenotype to a secretion-negative phenotype.
Figures
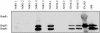
Similar articles
-
Type 3 secretion system effector genotype and secretion phenotype of longitudinally collected Pseudomonas aeruginosa isolates from young children diagnosed with cystic fibrosis following newborn screening.Clin Microbiol Infect. 2013 Mar;19(3):266-72. doi: 10.1111/j.1469-0691.2012.03770.x. Epub 2012 Feb 13. Clin Microbiol Infect. 2013. PMID: 22329595
-
Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa.Microbiology (Reading). 2001 Oct;147(Pt 10):2659-2669. doi: 10.1099/00221287-147-10-2659. Microbiology (Reading). 2001. PMID: 11577145
-
Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection.Infect Immun. 2005 Mar;73(3):1695-705. doi: 10.1128/IAI.73.3.1695-1705.2005. Infect Immun. 2005. PMID: 15731070 Free PMC article.
-
Immuno-modulatory functions of the type-3 secretion system and impacts on the pulmonary host defense: A role for ExoS of Pseudomonas aeruginosa in cystic fibrosis.Toxicon. 2018 Mar 1;143:68-73. doi: 10.1016/j.toxicon.2018.01.004. Epub 2018 Jan 12. Toxicon. 2018. PMID: 29339019 Review.
-
Polyagglutinable Pseudomonas aeruginosa from cystic fibrosis patients. A survey.APMIS Suppl. 1994;46:1-44. APMIS Suppl. 1994. PMID: 7811529 Review.
Cited by
-
Rampant Cheating by Pathogens?PLoS Pathog. 2016 Sep 8;12(9):e1005792. doi: 10.1371/journal.ppat.1005792. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27606630 Free PMC article. Review. No abstract available.
-
Positive signature-tagged mutagenesis in Pseudomonas aeruginosa: tracking patho-adaptive mutations promoting airways chronic infection.PLoS Pathog. 2011 Feb 3;7(2):e1001270. doi: 10.1371/journal.ppat.1001270. PLoS Pathog. 2011. PMID: 21304889 Free PMC article.
-
Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients.Mol Microbiol. 2007 Apr;64(2):512-33. doi: 10.1111/j.1365-2958.2007.05678.x. Mol Microbiol. 2007. PMID: 17493132 Free PMC article.
-
Absence of membrane phosphatidylcholine does not affect virulence and stress tolerance phenotypes in the opportunistic pathogen Pseudomonas aeruginosa.PLoS One. 2012;7(2):e30829. doi: 10.1371/journal.pone.0030829. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363496 Free PMC article.
-
Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis.Sci Rep. 2015 Jun 5;5:10932. doi: 10.1038/srep10932. Sci Rep. 2015. PMID: 26047320 Free PMC article.
References
-
- Abman, S. H., J. W. Ogle, R. J. Harbeck, N. Butler-Simon, K. B. Hammond, and F. J. Accurso. 1991. Early bacteriologic, immunologic, and clinical courses of young infants with cystic fibrosis identified by neonatal screening. J. Pediatr. 119:211-217. - PubMed
-
- Banwart, B., M. L. Splaingard, P. M. Farrell, M. J. Rock, P. L. Havens, J. Moss, M. E. Ehrmantraut, D. W. Frank, and J. T. Barbieri. 2002. Children with cystic fibrosis produce an immune response against exoenzyme S, a type III cytotoxin of Pseudomonas aeruginosa. J. Infect. Dis. 185:269-270. - PubMed
-
- Berthelot, P., I. Attree, P. Plesiat, J. Chabert, S. de Bentzmann, B. Pozzetto, and F. Grattard. 2003. Genotypic and phenotypic analysis of type III secretion system in a cohort of Pseudomonas aeruginosa bacteremia isolates: evidence for a possible association between O serotypes and exo genes. J. Infect. Dis. 188:512-518. - PubMed
-
- Bryan, L. E. 1989. Microbial persistence or phenotypic adaptation to antimicrobial agents: cystic fibrosis as an illustrative case, p. 411-420. In L. E. Bryan (ed.), Microbial resistance to drugs. Springer-Verlag, Berlin, Germany.
-
- Cheng, K., R. L. Smyth, J. R. Govan, C. Doherty, C. Winstanley, N. Denning, D. P. Heaf, H. van Saene, and C. A. Hart. 1996. Spread of beta-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 348:639-642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

