Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals
- PMID: 15529376
- PMCID: PMC4950930
- DOI: 10.1002/art.20601
Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals
Abstract
Objective: The mechanisms by which chondrocytes convert biomechanical signals into intracellular biochemical events are not well understood. In this study, we sought to determine the intracellular mechanisms of the magnitude-dependent actions of mechanical signals.
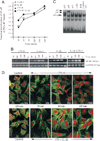
Methods: Chondrocytes isolated from rabbit articular cartilage were grown on flexible membranes. Cells were subjected to cyclic tensile strain (CTS) of various magnitudes in the presence or absence of interleukin-1beta (IL-1beta), which was used as a proinflammatory signal for designated time intervals. The regulation of NF-kappaB was measured by reverse transcriptase-polymerase chain reaction, electrophoretic mobility shift assay, and immunofluorescence.
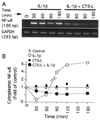
Results: CTS of low magnitudes (4-8% equibiaxial strain) was a potent inhibitor of IL-1beta-dependent NF-kappaB nuclear translocation. Cytoplasmic retention of NF-kappaB and reduction of its synthesis led to sustained suppression of proinflammatory gene induction. In contrast, proinflammatory signals generated by CTS of high magnitudes (15-18% equibiaxial strain) mimicked the actions of IL-1beta and induced rapid nuclear translocation of NF-kappaB subunits p65 and p50.
Conclusion: Magnitude-dependent signals of mechanical strain utilize the NF-kappaB transcription factors as common elements to abrogate or aggravate proinflammatory responses. Furthermore, the intracellular events induced by mechanical overload are similar to those that are initiated by proinflammatory cytokines in arthritis.
Figures


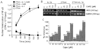
References
-
- Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19:242–249. - PubMed
-
- Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48:1292–1301. - PubMed
-
- Huang CY, Soltz MA, Kopacz M, Mow VC, Ateshian GA. Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng. 2003;125:84–93. - PubMed
-
- Buschmann MD, Kim YJ, Wong M, Frank E, Hunziker EB, Grodzinsky AJ. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

