Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry
- PMID: 15530413
- PMCID: PMC7092874
- DOI: 10.1016/j.bbrc.2004.10.052
Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry
Abstract
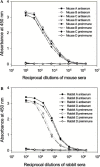
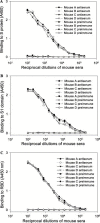
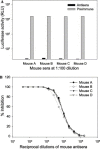
The only severe acute respiratory syndrome (SARS) vaccine currently being tested in clinical trial consists of inactivated severe acute respiratory syndrome-associate coronavirus (SARS-CoV). However, limited information is available about host immune responses induced by the inactivated SARS vaccine. In this study, we demonstrated that SARS-CoV inactivated by beta-propiolactone elicited high titers of antibodies in the immunized mice and rabbits that recognize the spike (S) protein, especially the receptor-binding domain (RBD) in the S1 region. The antisera from the immunized animals efficiently bound to the RBD and blocked binding of RBD to angiotensin-converting enzyme 2, the functional receptor on the susceptible cells for SARS-CoV. With a sensitive and quantitative single-cycle infection assay using pseudovirus bearing the SARS-CoV S protein, we demonstrated that mouse and rabbit antisera significantly inhibited S protein-mediated virus entry with mean 50% inhibitory titers of 1:7393 and 1:2060, respectively. These data suggest that the RBD of S protein is a major neutralization determinant in the inactivated SARS vaccine which can induce potent neutralizing antibodies to block SARS-CoV entry. However, caution should be taken in using the inactivated SARS-CoV as a vaccine since it may also cause harmful immune and/or inflammatory responses.
Figures
Similar articles
-
Identification of a critical neutralization determinant of severe acute respiratory syndrome (SARS)-associated coronavirus: importance for designing SARS vaccines.Virology. 2005 Mar 30;334(1):74-82. doi: 10.1016/j.virol.2005.01.034. Virology. 2005. PMID: 15749124 Free PMC article.
-
Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies.J Immunol. 2005 Apr 15;174(8):4908-15. doi: 10.4049/jimmunol.174.8.4908. J Immunol. 2005. PMID: 15814718
-
Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region.J Virol. 2005 Mar;79(5):2678-88. doi: 10.1128/JVI.79.5.2678-2688.2005. J Virol. 2005. PMID: 15708987 Free PMC article.
-
Vaccine design for severe acute respiratory syndrome coronavirus.Viral Immunol. 2005;18(2):327-32. doi: 10.1089/vim.2005.18.327. Viral Immunol. 2005. PMID: 16035944 Review.
-
SARS vaccine development.Emerg Infect Dis. 2005 Jul;11(7):1016-20. doi: 10.3201/1107.050219. Emerg Infect Dis. 2005. PMID: 16022774 Free PMC article. Review.
Cited by
-
Receptor-binding domain-specific human neutralizing monoclonal antibodies against SARS-CoV and SARS-CoV-2.Signal Transduct Target Ther. 2020 Sep 22;5(1):212. doi: 10.1038/s41392-020-00318-0. Signal Transduct Target Ther. 2020. PMID: 32963228 Free PMC article. Review.
-
Mucosal immunization with surface-displayed severe acute respiratory syndrome coronavirus spike protein on Lactobacillus casei induces neutralizing antibodies in mice.J Virol. 2006 Apr;80(8):4079-87. doi: 10.1128/JVI.80.8.4079-4087.2006. J Virol. 2006. PMID: 16571824 Free PMC article.
-
SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro.Virus Res. 2009 Jun;142(1-2):19-27. doi: 10.1016/j.virusres.2009.01.005. Epub 2009 Jan 29. Virus Res. 2009. PMID: 19185596 Free PMC article.
-
Two-way antigenic cross-reactivity between severe acute respiratory syndrome coronavirus (SARS-CoV) and group 1 animal CoVs is mediated through an antigenic site in the N-terminal region of the SARS-CoV nucleoprotein.J Virol. 2007 Dec;81(24):13365-77. doi: 10.1128/JVI.01169-07. Epub 2007 Oct 3. J Virol. 2007. PMID: 17913799 Free PMC article.
-
Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV).Perspect Med Virol. 2006;16:43-95. doi: 10.1016/S0168-7069(06)16004-8. Epub 2006 Nov 28. Perspect Med Virol. 2006. PMID: 32287586 Free PMC article. Review.
References
-
- Drosten C., Gunther S., Preiser W., van der W.S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. - PubMed
-
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. - PubMed
-
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. - PubMed
-
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

