Misfolded proteins, endoplasmic reticulum stress and neurodegeneration
- PMID: 15530777
- PMCID: PMC3970707
- DOI: 10.1016/j.ceb.2004.09.012
Misfolded proteins, endoplasmic reticulum stress and neurodegeneration
Abstract
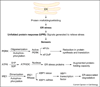
The accumulation of misfolded proteins (e.g. mutant or damaged proteins) triggers cellular stress responses that protect cells against the toxic buildup of such proteins. However, prolonged stress due to the buildup of these toxic proteins induces specific death pathways. Dissecting these pathways should be valuable in understanding the pathogenesis of, and ultimately in designing therapy for, neurodegenerative diseases that feature misfolded proteins.
Figures

References
-
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. - PubMed
-
- Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. - PubMed
-
- Kopito RR, Ron D. Conformational disease. Nat Cell Biol. 2000;2:E207–E209. - PubMed
-
- Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. - PubMed
-
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

