Characterization of plastidial thioredoxins from Arabidopsis belonging to the new y-type
- PMID: 15531707
- PMCID: PMC535839
- DOI: 10.1104/pp.104.052233
Characterization of plastidial thioredoxins from Arabidopsis belonging to the new y-type
Abstract
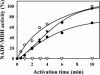
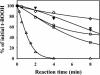
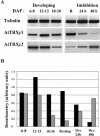
The plant plastidial thioredoxins (Trx) are involved in the light-dependent regulation of many enzymatic activities, owing to their thiol-disulfide interchange activity. Three different types of plastidial Trx have been identified and characterized so far: the m-, f-, and x-types. Recently, a new putative plastidial type, the y-type, was found. In this work the two isoforms of Trx y encoded by the nuclear genome of Arabidopsis (Arabidopsis thaliana) were characterized. The plastidial targeting of Trx y has been established by the expression of a TrxGFP fusion protein. Then both isoforms were produced as recombinant proteins in their putative mature forms and purified to characterize them by a biochemical approach. Their ability to activate two plastidial light-regulated enzymes, NADP-malate dehydrogenase (NADP-MDH) and fructose-1,6-bisphosphatase, was tested. Both Trx y were poor activators of fructose-1,6-bisphosphatase and NADP-MDH; however, a detailed study of the activation of NADP-MDH using site-directed mutants of its regulatory cysteines suggested that Trx y was able to reduce the less negative regulatory disulfide but not the more negative regulatory disulfide. This property probably results from the fact that Trx y has a less negative redox midpoint potential (-337 mV at pH 7.9) than thioredoxins f and m. The y-type Trxs were also the best substrate for the plastidial peroxiredoxin Q. Gene expression analysis showed that Trx y2 was mainly expressed in leaves and induced by light, whereas Trx y1 was mainly expressed in nonphotosynthetic organs, especially in seeds at a stage of major accumulation of storage lipids.
Figures




Similar articles
-
The Arabidopsis plastidial thioredoxins: new functions and new insights into specificity.J Biol Chem. 2003 Jun 27;278(26):23747-52. doi: 10.1074/jbc.M302077200. Epub 2003 Apr 21. J Biol Chem. 2003. PMID: 12707279
-
New insights into the reduction systems of plastidial thioredoxins point out the unique properties of thioredoxin z from Arabidopsis.J Exp Bot. 2012 Nov;63(18):6315-23. doi: 10.1093/jxb/ers283. Epub 2012 Oct 23. J Exp Bot. 2012. PMID: 23096001
-
Atypical thioredoxins in poplar: the glutathione-dependent thioredoxin-like 2.1 supports the activity of target enzymes possessing a single redox active cysteine.Plant Physiol. 2012 Jun;159(2):592-605. doi: 10.1104/pp.112.197723. Epub 2012 Apr 20. Plant Physiol. 2012. PMID: 22523226 Free PMC article.
-
Thioredoxin and glutaredoxin systems in plants: molecular mechanisms, crosstalks, and functional significance.Antioxid Redox Signal. 2012 Oct 15;17(8):1124-60. doi: 10.1089/ars.2011.4327. Epub 2012 Jun 8. Antioxid Redox Signal. 2012. PMID: 22531002 Review.
-
Regulatory roles of thioredoxin in oxidative stress-induced cellular responses.Redox Rep. 2001;6(5):289-95. doi: 10.1179/135100001101536427. Redox Rep. 2001. PMID: 11778846 Review.
Cited by
-
Reactive oxygen species, oxidative signaling and the regulation of photosynthesis.Environ Exp Bot. 2018 Oct;154:134-142. doi: 10.1016/j.envexpbot.2018.05.003. Environ Exp Bot. 2018. PMID: 30283160 Free PMC article. Review.
-
Oxidative regulation of chloroplast enzymes by thioredoxin and thioredoxin-like proteins in Arabidopsis thaliana.Proc Natl Acad Sci U S A. 2021 Dec 21;118(51):e2114952118. doi: 10.1073/pnas.2114952118. Proc Natl Acad Sci U S A. 2021. PMID: 34907017 Free PMC article.
-
Plastid thioredoxins: a "one-for-all" redox-signaling system in plants.Front Plant Sci. 2013 Nov 21;4:463. doi: 10.3389/fpls.2013.00463. Front Plant Sci. 2013. PMID: 24319449 Free PMC article. Review.
-
Exploring the Functional Relationship between y-Type Thioredoxins and 2-Cys Peroxiredoxins in Arabidopsis Chloroplasts.Antioxidants (Basel). 2020 Oct 31;9(11):1072. doi: 10.3390/antiox9111072. Antioxidants (Basel). 2020. PMID: 33142810 Free PMC article.
-
New In Vivo Approach to Broaden the Thioredoxin Family Interactome in Chloroplasts.Antioxidants (Basel). 2022 Oct 4;11(10):1979. doi: 10.3390/antiox11101979. Antioxidants (Basel). 2022. PMID: 36290702 Free PMC article.
References
-
- Baier M, Dietz K-J (1997) The plant 2-Cys peroxiredoxin BAS1 is a nuclear-encoded chloroplast protein: its expressional regulation, phylogenetic origin, and implications for its specific physiological function in plants. Plant J 12: 179–190 - PubMed
-
- Buchanan BB (1980) Role of light in the regulation of chloroplast enzymes. Annu Rev Plant Physiol 31: 341–374
-
- Collin V, Issakidis-Bourguet E, Marchand C, Hirasawa M, Lancelin JM, Knaff DB, Miginiac-Maslow M (2003) The Arabidopsis plastidial thioredoxins: new functions and new insights into specificity. J Biol Chem 278: 23747–23752 - PubMed
-
- Dietz KJ (2003) Plant peroxiredoxins. Annu Rev Plant Biol 54: 93–107 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

