Nanoscale features of fibronectin fibrillogenesis depend on protein-substrate interaction and cytoskeleton structure
- PMID: 15533920
- PMCID: PMC1305030
- DOI: 10.1529/biophysj.104.048074
Nanoscale features of fibronectin fibrillogenesis depend on protein-substrate interaction and cytoskeleton structure
Abstract
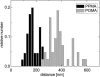
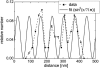
Cell-reorganized fibronectin layers on polymer films providing a gradation of the binding strength between protein and substrate were analyzed by combined fluorescence and scanning force microscopy. The nanoscale fibronectin patterns exhibited paired parallel fibrils with characteristic spacings of 156, 233, 304, and 373 nm. These spacings depend on the interaction of fibronectin with the substrate: at enhanced fibronectin-substrate anchorage the cells form larger stress fibers, which are assembled by alpha-actinin cross-linked pairs of actin filaments subunits at the focal adhesions. A ubiquitous repeating unit of approximately 71 nm was found within these characteristic distances. We conclude that the dimensions of the actin stress fibers reflect the binding strength of fibronectin to the polymer substrate and act--in turn--as a template for the reorganization of fibronectin into surface-bound nanofibrils with characteristic spacings. This explanation was confirmed by data showing the alpha-actinin/fibronectin colocalization.
Figures







Similar articles
-
Molecular assembly and biological activity of a recombinant fragment of fibronectin (FNIII(7-10)) on poly(ethyl acrylate).Colloids Surf B Biointerfaces. 2010 Jul 1;78(2):310-6. doi: 10.1016/j.colsurfb.2010.03.019. Epub 2010 Mar 29. Colloids Surf B Biointerfaces. 2010. PMID: 20409696
-
Analysis of the biological response of endothelial and fibroblast cells cultured on synthetic scaffolds with various hydrophilic/hydrophobic ratios: influence of fibronectin adsorption and conformation.Tissue Eng Part A. 2009 Jun;15(6):1331-41. doi: 10.1089/ten.tea.2008.0146. Tissue Eng Part A. 2009. PMID: 18976156
-
Biological activity of the substrate-induced fibronectin network: insight into the third dimension through electrospun fibers.Langmuir. 2009 Sep 15;25(18):10893-900. doi: 10.1021/la9012203. Langmuir. 2009. PMID: 19735141
-
Nanopatterning of fibronectin and the influence of integrin clustering on endothelial cell spreading and proliferation.J Biomed Mater Res A. 2008 Oct;87(1):176-95. doi: 10.1002/jbm.a.31725. J Biomed Mater Res A. 2008. PMID: 18085648
-
Fibronectin fibrillogenesis, a cell-mediated matrix assembly process.Matrix Biol. 2005 Sep;24(6):389-99. doi: 10.1016/j.matbio.2005.06.008. Matrix Biol. 2005. PMID: 16061370 Review.
Cited by
-
A comparative study on in vitro osteogenic priming potential of electron spun scaffold PLLA/HA/Col, PLLA/HA, and PLLA/Col for tissue engineering application.PLoS One. 2014 Aug 20;9(8):e104389. doi: 10.1371/journal.pone.0104389. eCollection 2014. PLoS One. 2014. PMID: 25140798 Free PMC article.
-
Immobilization of growth factors on solid supports for the modulation of stem cell fate.Nat Protoc. 2010 Jun;5(6):1042-50. doi: 10.1038/nprot.2010.70. Epub 2010 May 13. Nat Protoc. 2010. PMID: 20539280
-
The relative importance of topography and RGD ligand density for endothelial cell adhesion.PLoS One. 2011;6(7):e21869. doi: 10.1371/journal.pone.0021869. Epub 2011 Jul 11. PLoS One. 2011. PMID: 21779342 Free PMC article.
-
Fibronectin fibril pattern displays the force balance of cell-matrix adhesion.Eur Biophys J. 2005 Nov;34(8):1049-56. doi: 10.1007/s00249-005-0490-z. Epub 2005 Jul 12. Eur Biophys J. 2005. PMID: 16010568
-
The role of surface chemistry-induced cell characteristics on nonviral gene delivery to mouse fibroblasts.J Biol Eng. 2012 Sep 11;6(1):17. doi: 10.1186/1754-1611-6-17. J Biol Eng. 2012. PMID: 22967455 Free PMC article.
References
-
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. 1994. Molecular Biology of the Cell, 3rd ed. Garland Publishing, New York.
-
- Altankov, G., F. Grinnell, and T. Groth. 1996. Studies on the biocompatibility of materials: fibroblast reorganization of substratumbound fibronectin on surfaces varying in wettability. J. Biomed. Mater. Res. 30:385–391. - PubMed
-
- Balaban, N. Q., U. S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–473. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

