Nanoscale features of fibronectin fibrillogenesis depend on protein-substrate interaction and cytoskeleton structure
- PMID: 15533920
- PMCID: PMC1305030
- DOI: 10.1529/biophysj.104.048074
Nanoscale features of fibronectin fibrillogenesis depend on protein-substrate interaction and cytoskeleton structure
Abstract
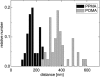
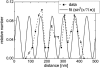
Cell-reorganized fibronectin layers on polymer films providing a gradation of the binding strength between protein and substrate were analyzed by combined fluorescence and scanning force microscopy. The nanoscale fibronectin patterns exhibited paired parallel fibrils with characteristic spacings of 156, 233, 304, and 373 nm. These spacings depend on the interaction of fibronectin with the substrate: at enhanced fibronectin-substrate anchorage the cells form larger stress fibers, which are assembled by alpha-actinin cross-linked pairs of actin filaments subunits at the focal adhesions. A ubiquitous repeating unit of approximately 71 nm was found within these characteristic distances. We conclude that the dimensions of the actin stress fibers reflect the binding strength of fibronectin to the polymer substrate and act--in turn--as a template for the reorganization of fibronectin into surface-bound nanofibrils with characteristic spacings. This explanation was confirmed by data showing the alpha-actinin/fibronectin colocalization.
Figures







References
-
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. 1994. Molecular Biology of the Cell, 3rd ed. Garland Publishing, New York.
-
- Altankov, G., F. Grinnell, and T. Groth. 1996. Studies on the biocompatibility of materials: fibroblast reorganization of substratumbound fibronectin on surfaces varying in wettability. J. Biomed. Mater. Res. 30:385–391. - PubMed
-
- Balaban, N. Q., U. S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–473. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

