Interpreting second-harmonic generation images of collagen I fibrils
- PMID: 15533922
- PMCID: PMC1305140
- DOI: 10.1529/biophysj.104.047308
Interpreting second-harmonic generation images of collagen I fibrils
Abstract
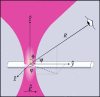
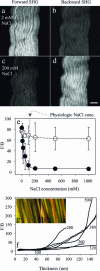
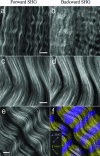
Fibrillar collagen, being highly noncentrosymmetric, possesses a tremendous nonlinear susceptibility. As a result, second-harmonic generation (SHG) microscopy of collagen produces extremely bright and robust signals, providing an invaluable tool for imaging tissue structure with submicron resolution. Here we discuss fundamental principles governing SHG phase matching with the tightly focusing optics used in microscopy. Their application to collagen imaging yields several biophysical features characteristic of native collagen structure: SHG radiates from the shell of a collagen fibril, rather than from its bulk. This SHG shell may correspond to the supporting element of the fibril. Physiologically relevant changes in solution ionic strength alter the ratio of forward-to-backward propagating SHG, implying a resulting change in the SHG shell thickness. Fibrillogenesis can be resolved in immature tissue by directly imaging backward-propagating SHG. Such findings are crucial to the design and development of forthcoming diagnostic and research tools.
Figures


References
-
- Barad, Y., H. Eisenberg, M. Horowitz, and Y. Silberberg. 1997. Nonlinear scanning laser microscopy by third harmonic generation. Appl. Phys. Lett. 70:922–924.
-
- Birk, D. E., and T. F. Linsenmayer. 1994. Collagen fibril assembly, deposition, and organization into tissue-specific matrices. In Extracellular Matrix Assembly and Structure. P. D. Yurchenco, D. E. Birk, and R. P. Mecham, editors. Academic Press, San Diego, CA. 91–128.
-
- Born, M., and E. Wolf. 1980. Principles of Optics. Pergamon Press, New York, NY.
-
- Boyd, R. W. 1992. Nonlinear Optics. Academic Press, Boston, MA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

