Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement
- PMID: 15534002
- PMCID: PMC2172490
- DOI: 10.1083/jcb.200406177
Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement
Abstract
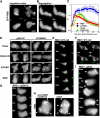
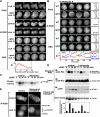
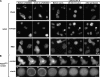
During chemotaxis, receptors and heterotrimeric G-protein subunits are distributed and activated almost uniformly along the cell membrane, whereas PI(3,4,5)P(3), the product of phosphatidylinositol 3-kinase (PI3K), accumulates locally at the leading edge. The key intermediate event that creates this strong PI(3,4,5)P(3) asymmetry remains unclear. Here, we show that Ras is rapidly and transiently activated in response to chemoattractant stimulation and regulates PI3K activity. Ras activation occurs at the leading edge of chemotaxing cells, and this local activation is independent of the F-actin cytoskeleton, whereas PI3K localization is dependent on F-actin polymerization. Inhibition of Ras results in severe defects in directional movement, indicating that Ras is an upstream component of the cell's compass. These results support a mechanism by which localized Ras activation mediates leading edge formation through activation of basal PI3K present on the plasma membrane and other Ras effectors required for chemotaxis. A feedback loop, mediated through localized F-actin polymerization, recruits cytosolic PI3K to the leading edge to amplify the signal.
Figures





References
-
- Chan, T.O., U. Rodeck, A.M. Chan, A.C. Kimmelman, S.E. Rittenhouse, G. Panayotou, and P.N. Tsichlis. 2002. Small GTPases and tyrosine kinases coregulate a molecular switch in the phosphoinositide 3-kinase regulatory subunit. Cancer Cell. 1:181–191. - PubMed
-
- Chiu, V., T. Bivona, A. Hach, J. Sajous, J. Silletti, H. Wiener, R.N. Johnson, A. Cox, and M. Philips. 2002. Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell Biol. 4:343–350. - PubMed
-
- Chung, C., S. Funamoto, and R. Firtel. 2001. a. Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem. Sci. 26:557–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

