Remote hot spots mediate protein substrate recognition for the Cdc25 phosphatase
- PMID: 15534213
- PMCID: PMC534539
- DOI: 10.1073/pnas.0407663101
Remote hot spots mediate protein substrate recognition for the Cdc25 phosphatase
Abstract
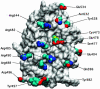
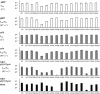
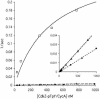
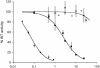
Cdc25B is a phosphatase that catalyzes the dephosphorylation and activation of the cyclin-dependent kinases, thus driving cell cycle progression. We have identified two residues, R488 and Y497, located >20 A from the active site, that mediate protein substrate recognition without affecting activity toward small-molecule substrates. Injection of Cdc25B wild-type but not the R488L or Y497A variants induces germinal vesicle breakdown and cyclin-dependent kinase activation in Xenopus oocytes. The conditional knockout of the cdc25 homolog (mih1) in Saccharomyces cerevisiae can be complemented by the wild type but not by the hot spot variants, indicating that protein substrate recognition by the Cdc25 phosphatases is an essential and evolutionarily conserved feature.
Figures


References
-
- Bogan, A. A. & Thorn, K. S. (1998) J. Mol. Biol. 280, 1–9. - PubMed
-
- Clackson, T. & Wells, J. A. (1995) Science 267, 383–386. - PubMed
-
- Schreiber, S. & Fersht, A. R. (1995) J. Mol. Biol. 248, 478–486. - PubMed
-
- Nilsson, I. & Hoffmann, I. (2000) Prog. Cell Cycle Res. 4, 107–114. - PubMed
-
- Kristjánsdóttir, K. & Rudolph, J. (2004) Chem. Biol. 11, 1043–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

