Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex
- PMID: 15534214
- PMCID: PMC528983
- DOI: 10.1073/pnas.0407586101
Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex
Abstract
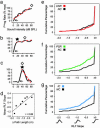
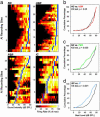
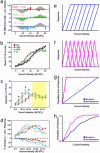
Since the dawn of experimental psychology, researchers have sought an understanding of the fundamental relationship between the amplitude of sensory stimuli and the magnitudes of their perceptual representations. Contemporary theories support the view that magnitude is encoded by a linear increase in firing rate established in the primary afferent pathways. In the present study, we have investigated sound intensity coding in the rat primary auditory cortex (AI) and describe its plasticity by following paired stimulus reinforcement and instrumental conditioning paradigms. In trained animals, population-response strengths in AI became more strongly nonlinear with increasing stimulus intensity. Individual AI responses became selective to more restricted ranges of sound intensities and, as a population, represented a broader range of preferred sound levels. These experiments demonstrate that the representation of stimulus magnitude can be powerfully reshaped by associative learning processes and suggest that the code for sound intensity within AI can be derived from intensity-tuned neurons that change, rather than simply increase, their firing rates in proportion to increases in sound intensity.
Figures

References
-
- Stevens, S. S. (1975) Psychophysics: Introduction to Its Perceptual, Neural, and Social Prospects (Wiley, New York).
-
- Werner, G. & Mountcastle, V. B. (1965) J. Neurophysiol. 28, 359-397. - PubMed
-
- Mountcastle, V. B., Poggio, G. F. & Werner, G. (1963) J. Neurophysiol. 26, 807-834. - PubMed
-
- Johnson, K. O., Hsiao, S. S. & Blake, D. T. (1996) in Somesthesis and the Neurobiology of the Somatosensory Cortex, eds. Franzen, O., Johansson, R. & Terenius, L. (Birkhäuser, Basel), pp. 213-228.
-
- Moore, B. J. (1995) Hearing (Academic, New York).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

