Replication-mediated instability of the GAA triplet repeat mutation in Friedreich ataxia
- PMID: 15534367
- PMCID: PMC528813
- DOI: 10.1093/nar/gkh933
Replication-mediated instability of the GAA triplet repeat mutation in Friedreich ataxia
Abstract
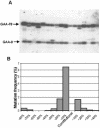
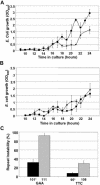
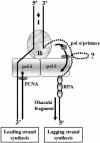
Friedreich ataxia is caused by the expansion of a polymorphic and unstable GAA triplet repeat in the FRDA gene, but the mechanisms for its instability are poorly understood. Replication of (GAA*TTC)n sequences (9-105 triplets) in plasmids propagated in Escherichia coli displayed length- and orientation-dependent instability. There were small length variations upon replication in both orientations, but large contractions were frequently observed when GAA was the lagging strand template. DNA replication was also significantly slower in this orientation. To evaluate the physiological relevance of our findings, we analyzed peripheral leukocytes from human subjects carrying repeats of similar length (8-107 triplets). Analysis of 9400 somatic FRDA molecules using small-pool PCR revealed a similar mutational spectrum, including large contractions. The threshold length for the initiation of somatic instability in vivo was between 40 and 44 triplets, corresponding to the length of a eukaryotic Okazaki fragment. Consistent with the stabilization of premutation alleles during germline transmission, we also found that instability of somatic cells in vivo and repeats propagated in E.coli were abrogated by (GAGGAA)n hexanucleotide interruptions. Our data demonstrate that the GAA triplet repeat mutation in Friedreich ataxia is destabilized, frequently undergoing large contractions, during DNA replication.
Figures
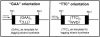

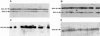

References
-
- Campuzano V., Montermini,L., Moltó,M.D., Pianese,L., Cossée,M., Cavalcanti,E., Monrós,F., Rodius,F., Duclos,F., Monticelli,A. et al. (1996) Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science, 271, 1423–1427. - PubMed
-
- Bidichandani S.I. and Ashizawa,T. (2002) Friedreich ataxia, GeneReviews: Genetic Disease Online Reviews of GeneTests-GeneClinics. Copyright University of Washington, Seattle, WA, USA.
-
- Montermini L., Andermann,E., Labuda,M., Richter,A., Pandolfo,M., Cavalcanti,F., Pianese,L., Iodice,L., Farina,G., Monticelli,A. et al. (1997) The Friedreich ataxia GAA triplet repeat: premutation and normal alleles. Hum. Mol. Genet., 6, 1261–1266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

