Inherent growth advantage of (pre)malignant hepatocytes associated with nuclear translocation of pro-transforming growth factor alpha
- PMID: 15534611
- PMCID: PMC2409777
- DOI: 10.1038/sj.bjc.6602191
Inherent growth advantage of (pre)malignant hepatocytes associated with nuclear translocation of pro-transforming growth factor alpha
Abstract
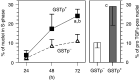
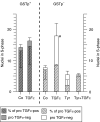
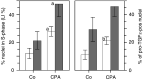
The pro-peptide of transforming growth factor alpha (proTGFalpha) was recently found in hepatocyte nuclei preparing for DNA replication, which suggests a role of nuclear proTGFalpha for mitogenic signalling. This study investigates whether the nuclear occurrence of the pro-peptide is involved in the altered growth regulation of (pre)malignant hepatocytes. In human hepatocarcinogenesis, the incidence of proTGFalpha-positive and replicating nuclei gradually increased from normal liver, to dysplastic nodules, to hepatocellular carcinoma. ProTGFalpha-positive nuclei almost always were in DNA synthesis. Also, in rat hepatocarcinogenesis, proTGFalpha-positive nuclei occurred in (pre)malignant hepatocytes at significantly higher incidences than in unaltered hepatocytes. For functional studies unaltered (GSTp(-)) and premalignant (GSTp(+)) rat hepatocytes were isolated by collagenase perfusion and cultivated. Again, DNA synthesis occurred almost exclusively in proTGFalpha-positive nuclei. GSTp(+) hepatocytes showed an approximately 3-fold higher frequency of proTGFalpha-positive nuclei and DNA replication than GSTp(-) cells. Treatment of cultures with the mitogen cyproterone acetate (CPA) elevated the incidence of proTGFalpha-positive nuclei and DNA synthesis in parallel. Conversely, transforming growth factor beta1 (TGFbeta1) lowered both. These effects of CPA and TGFbeta1 were significantly more pronounced in GSTp(+) than in GSTp(-) hepatocytes. In conclusion, nuclear translocation of proTGFalpha increases in the course of hepatocarcinogenesis and appears to be involved in the inherent growth advantage of (pre)malignant hepatocytes.
Figures




Similar articles
-
Role of transforming growth factor alpha and prostaglandins in preferential growth of preneoplastic rat hepatocytes.Carcinogenesis. 2001 Aug;22(8):1247-56. doi: 10.1093/carcin/22.8.1247. Carcinogenesis. 2001. PMID: 11470756
-
Initiated rat hepatocytes in primary culture: a novel tool to study alterations in growth control during the first stage of carcinogenesis.Carcinogenesis. 2000 Jan;21(1):79-86. doi: 10.1093/carcin/21.1.79. Carcinogenesis. 2000. PMID: 10607737
-
A novel mechanism for mitogenic signaling via pro-transforming growth factor alpha within hepatocyte nuclei.Hepatology. 2002 Jun;35(6):1372-80. doi: 10.1053/jhep.2002.33203. Hepatology. 2002. PMID: 12029622
-
Induction of DNA synthesis in primary mouse hepatocytes is associated with nuclear pro-transforming growth factor alpha and erbb-1 and is independent of c-jun.Carcinogenesis. 2003 May;24(5):835-41. doi: 10.1093/carcin/bgg027. Carcinogenesis. 2003. PMID: 12771026
-
Interferon alfa-2b triggers transforming growth factor-beta-induced apoptosis on preneoplasticliver.Ann Hepatol. 2006 Oct-Dec;5(4):244-50. Ann Hepatol. 2006. PMID: 17153763 Review.
Cited by
-
Validation of miR-20a as a Tumor Suppressor Gene in Liver Carcinoma Using Hepatocyte-Specific Hyperactive piggyBac Transposons.Mol Ther Nucleic Acids. 2020 Mar 6;19:1309-1329. doi: 10.1016/j.omtn.2020.01.015. Epub 2020 Jan 22. Mol Ther Nucleic Acids. 2020. PMID: 32160703 Free PMC article.
References
-
- Deguchi T, Pitot HC (1995) Expression of c-myc in altered hepatic foci induced in rats by various single doses of diethylnitrosamine and promotion by 0,05% phenobarbital. Mol Carcinogen 14: 152–159 - PubMed
-
- DeGunst MCM, Luebeck EG (1998) A method for parametric estimation of the number and size distribution of cell clusters from observations in a section plane. Biometrics 54: 100–112 - PubMed
-
- Edmondson HA, Steiner PE (1954) Primary carcinoma of the liver: a study of 100 cases among 48.900 necropsies. Cancer 7: 462–503 - PubMed
-
- El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340: 745–750 - PubMed
-
- Fiel MI, Min A, Gerber MA, Faire B, Schwartz M, Thung SN (1996) Hepatocellular carcinoma in long-term oral contraceptive use. Liver 16: 372–376 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

