The effect of adenovirus expressing wild-type p53 on 5-fluorouracil chemosensitivity is related to p53 status in pancreatic cancer cell lines
- PMID: 15534911
- PMCID: PMC4611997
- DOI: 10.3748/wjg.v10.i24.3583
The effect of adenovirus expressing wild-type p53 on 5-fluorouracil chemosensitivity is related to p53 status in pancreatic cancer cell lines
Abstract
Aim: There are conflicting data about p53 function on cellular sensitivity to the cytotoxic action of 5-fluorouracil (5-FU). Therefore the objective of this study was to determine the combined effects of adenovirus-mediated wild-type (wt) p53 gene transfer and 5-FU chemotherapy on pancreatic cancer cells with different p53 gene status.
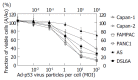
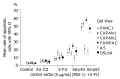
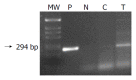
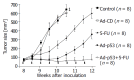
Methods: Human pancreatic cancer cell lines Capan-1(p53mut), Capan-2(p53wt), FAMPAC(p53mut), PANC1(p53mut), and rat pancreatic cancer cell lines AS(p53wt) and DSL6A(p53null) were used for in vitro studies. Following infection with different ratios of Ad-p53-particles (MOI) in combination with 5-FU, proliferation of tumor cells and apoptosis were quantified by cell proliferation assay (WST-1) and FACS (PI-staining). In addition, DSL6A syngeneic pancreatic tumor cells were inoculated subcutaneously in to Lewis rats for in vivo studies. Tumor size, apoptosis (TUNEL) and survival were determined.
Results: Ad-p53 gene transfer combined with 5-FU significantly inhibited tumor cell proliferation and substantially enhanced apoptosis in all four cell lines with an alteration in the p53 gene compared to those two cell lines containing wt-p53. In vivo experiments showed the most effective tumor regression in animals treated with Ad-p53 plus 5-FU. Both in vitro and in vivo analyses revealed that a sublethal dose of Ad-p53 augmented the apoptotic response induced by 5-FU.
Conclusion: Our results suggest that Ad-p53 may synergistically enhance 5-FU-chemosensitivity most strikingly in pancreatic cancer cells lacking p53 function. These findings illustrate that the anticancer efficacy of this combination treatment is dependent on the p53 gene status of the target tumor cells.
Figures

Similar articles
-
Influence of p53 status on radiation and 5-flourouracil synergy in pancreatic cancer cells.Anticancer Res. 2002 Mar-Apr;22(2A):825-30. Anticancer Res. 2002. PMID: 12014658
-
5-Fluorouracil or gemcitabine combined with adenoviral-mediated reintroduction of p16INK4A greatly enhanced cytotoxicity in Panc-1 pancreatic adenocarcinoma cells.J Gene Med. 2004 May;6(5):514-25. doi: 10.1002/jgm.540. J Gene Med. 2004. PMID: 15133762
-
Prevention of chemotherapy-related toxic side effects by infection with adeno-associated virus type 2.Int J Cancer. 2002 Aug 10;100(5):606-14. doi: 10.1002/ijc.10152. Int J Cancer. 2002. PMID: 12124812
-
Adenovirus-mediated p53 gene therapy for human gliomas.Neurosurgery. 1999 Nov;45(5):1093-104. doi: 10.1097/00006123-199911000-00016. Neurosurgery. 1999. PMID: 10549925 Review.
-
Optimisation of replication-selective oncolytic adenoviral mutants in combination with chemotherapeutics.J BUON. 2009 Sep;14 Suppl 1:S61-7. J BUON. 2009. PMID: 19785071 Review.
Cited by
-
Biological activity of PtIV prodrugs triggered by riboflavin-mediated bioorthogonal photocatalysis.Sci Rep. 2018 Nov 21;8(1):17198. doi: 10.1038/s41598-018-35655-2. Sci Rep. 2018. PMID: 30464209 Free PMC article.
-
The p53 reactivator PRIMA-1MET synergises with 5-fluorouracil to induce apoptosis in pancreatic cancer cells.Invest New Drugs. 2023 Aug;41(4):587-595. doi: 10.1007/s10637-023-01380-5. Epub 2023 Jul 4. Invest New Drugs. 2023. PMID: 37402008
-
Gene medicine for cancer treatment: commercially available medicine and accumulated clinical data in China.Drug Des Devel Ther. 2009 Feb 6;2:115-22. doi: 10.2147/dddt.s3535. Drug Des Devel Ther. 2009. PMID: 19920899 Free PMC article.
-
Bugs and drugs: oncolytic virotherapy in combination with chemotherapy.Curr Pharm Biotechnol. 2012 Jul;13(9):1817-33. doi: 10.2174/138920112800958850. Curr Pharm Biotechnol. 2012. PMID: 21740354 Free PMC article. Review.
-
Hsp70 response to 5-fluorouracil treatment in human colon cancer cell lines.Int J Colorectal Dis. 2007 Oct;22(10):1201-8. doi: 10.1007/s00384-007-0307-x. Epub 2007 Mar 28. Int J Colorectal Dis. 2007. PMID: 17390142
References
-
- Ghaneh P, Kawesha A, Evans JD, Neoptolemos JP. Molecular prognostic markers in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2002;9:1–11. - PubMed
-
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. - PubMed
-
- Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. - PubMed
-
- Lenz HJ, Hayashi K, Salonga D, Danenberg KD, Danenberg PV, Metzger R, Banerjee D, Bertino JR, Groshen S, Leichman LP, et al. p53 point mutations and thymidylate synthase messenger RNA levels in disseminated colorectal cancer: an analysis of response and survival. Clin Cancer Res. 1998;4:1243–1250. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

