Characterization of carnitine and fatty acid metabolism in the long-chain acyl-CoA dehydrogenase-deficient mouse
- PMID: 15535801
- PMCID: PMC1134946
- DOI: 10.1042/BJ20041489
Characterization of carnitine and fatty acid metabolism in the long-chain acyl-CoA dehydrogenase-deficient mouse
Abstract
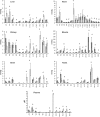
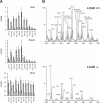
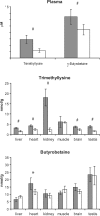
In the present paper, we describe a novel method which enables the analysis of tissue acylcarnitines and carnitine biosynthesis intermediates in the same sample. This method was used to investigate the carnitine and fatty acid metabolism in wild-type and LCAD-/- (long-chain acyl-CoA dehydrogenase-deficient) mice. In agreement with previous results in plasma and bile, we found accumulation of the characteristic C14:1-acylcarnitine in all investigated tissues from LCAD-/- mice. Surprisingly, quantitatively relevant levels of 3-hydroxyacylcarnitines were found to be present in heart, muscle and brain in wild-type mice, suggesting that, in these tissues, long-chain 3-hydroxyacyl-CoA dehydrogenase is rate-limiting for mitochondrial beta-oxidation. The 3-hydroxyacylcarnitines were absent in LCAD-/- tissues, indicating that, in this situation, the beta-oxidation flux is limited by the LCAD deficiency. A profound deficiency of acetylcarnitine was observed in LCAD-/- hearts, which most likely corresponds with low cardiac levels of acetyl-CoA. Since there was no carnitine deficiency and only a marginal elevation of potentially cardiotoxic acylcarnitines, we conclude from these data that the cardiomyopathy in the LCAD-/- mouse is caused primarily by a severe energy deficiency in the heart, stressing the important role of LCAD in cardiac fatty acid metabolism in the mouse.
Figures


References
-
- McGarry J. D., Brown N. F. The mitochondrial carnitine palmitoyltransferase system: from concept to molecular analysis. Eur. J. Biochem. 1997;244:1–14. - PubMed
-
- Ramsay R. R., Gandour R. D., van der Leij F. R. Molecular enzymology of carnitine transfer and transport. Biochim. Biophys. Acta. 2001;1546:21–43. - PubMed
-
- Rinaldo P. Fatty acid transport and mitochondrial oxidation disorders. Semin. Liver Dis. 2001;21:489–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

