The origins of estrogen receptor alpha-positive and estrogen receptor alpha-negative human breast cancer
- PMID: 15535853
- PMCID: PMC1064085
- DOI: 10.1186/bcr938
The origins of estrogen receptor alpha-positive and estrogen receptor alpha-negative human breast cancer
Abstract
Current hormonal therapies have benefited millions of patients with breast cancer. Their success, however, is often temporary and limited to a subset of patients whose tumors express estrogen receptor alpha (ER). The therapies are entirely ineffective in ER-negative disease. Recent studies suggest that there are many biological pathways and alterations involved in determining whether ER is expressed and how it is regulated during breast cancer evolution. Improving hormonal therapies, in addition to perfecting current strategies, will also target these newly discovered pathways and alterations, and others yet to be found. The present commentary will briefly highlight a few important observations and unanswered questions regarding ER status and growth regulation during breast cancer evolution, which hopefully will help to stimulate new thinking and progress in this important area of medial research.
Figures


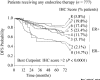

References
-
- Osborne CK, Ravdin PM. Adjuvant systemic therapy of primary breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editor. In Diseases of the Breast. 2. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 599–632.
-
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, and other NSABP Investigators et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. - DOI - PubMed
-
- Brown P, Fuqua S, Allred C. Pathogenesis of estrogen receptor positive and negative breast cancer. In: Ethier SP, editor. In Contemporary Endocrinology: Endocrine Oncology. Totowa, NJ: Humana Press; 2001. pp. 49–68.
-
- Allred DC, Mohsin SK, Fuqua SAW. Histological and biological evolution of human premalignant breast disease. Endocrine-Relat Cancer. 2001;8:47–61. - PubMed
-
- Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. - PubMed

