Alpha-synuclein structures from fluorescence energy-transfer kinetics: implications for the role of the protein in Parkinson's disease
- PMID: 15536128
- PMCID: PMC534538
- DOI: 10.1073/pnas.0407307101
Alpha-synuclein structures from fluorescence energy-transfer kinetics: implications for the role of the protein in Parkinson's disease
Abstract
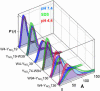
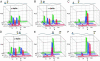
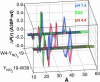
Parkinson's disease is associated with the deposition and accumulation of alpha-synuclein fibrils in the brain. A30P and A53T mutations have been linked to the early-onset familial disease state. Time-resolved tryptophan fluorescence energy-transfer measurements have been used to probe the structures of pseudo-wild-type and mutant (A30P) alpha-synucleins at physiological pH (7.4), in acidic pH (4.4) solutions, and in the presence of SDS micelles, a membrane mimic. Fluorescent donor-energy acceptor (DA) distance distributions for six different tryptophan/3-nitro-tyrosine pairs reveal the presence of compact, intermediate, and extended conformations of the protein. CD spectra indicate that the protein develops substantial helical structure in the presence of SDS micelles. DA distributions show that micelles induce compaction in the N-terminal region and expansion of the acidic C terminus. In acidic solutions, there is an increased population of collapsed structures in the C-terminal region. Energy-transfer measurements demonstrate that the average DA distances for the W4-Y19 and Y19-W39 pairs are longer in one of the two disease-related mutants (A30P).
Figures
Similar articles
-
Effect of familial Parkinson's disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein.Biochemistry. 2001 Sep 25;40(38):11604-13. doi: 10.1021/bi010616g. Biochemistry. 2001. PMID: 11560511
-
Conformational behavior of human alpha-synuclein is modulated by familial Parkinson's disease point mutations A30P and A53T.Neurotoxicology. 2002 Oct;23(4-5):553-67. doi: 10.1016/s0161-813x(02)00066-9. Neurotoxicology. 2002. PMID: 12428728
-
Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy.Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):571-6. doi: 10.1073/pnas.97.2.571. Proc Natl Acad Sci U S A. 2000. PMID: 10639120 Free PMC article.
-
Defective membrane interactions of familial Parkinson's disease mutant A30P alpha-synuclein.J Mol Biol. 2002 Jan 25;315(4):799-807. doi: 10.1006/jmbi.2001.5269. J Mol Biol. 2002. PMID: 11812148
-
Structure/function in neuroprotection and apoptosis.Ann Neurol. 1998 Sep;44(3 Suppl 1):S65-71. doi: 10.1002/ana.410440711. Ann Neurol. 1998. PMID: 9749576 Review.
Cited by
-
Are Charge-State Distributions a Reliable Tool Describing Molecular Ensembles of Intrinsically Disordered Proteins by Native MS?J Am Soc Mass Spectrom. 2017 Jan;28(1):21-28. doi: 10.1007/s13361-016-1490-1. Epub 2016 Oct 11. J Am Soc Mass Spectrom. 2017. PMID: 27730522 Review.
-
α-Synuclein: Experimental Pathology.Cold Spring Harb Perspect Med. 2016 Sep 1;6(9):a024273. doi: 10.1101/cshperspect.a024273. Cold Spring Harb Perspect Med. 2016. PMID: 27481772 Free PMC article. Review.
-
Early aggregation steps in alpha-synuclein as measured by FCS and FRET: evidence for a contagious conformational change.Biophys J. 2010 Apr 7;98(7):1302-11. doi: 10.1016/j.bpj.2009.12.4290. Biophys J. 2010. PMID: 20371330 Free PMC article.
-
Copper(II) Binding to the Intrinsically Disordered C-Terminal Peptide of SARS-CoV-2 Virulence Factor Nsp1.Inorg Chem. 2022 Jun 20;61(24):8992-8996. doi: 10.1021/acs.inorgchem.2c01329. Epub 2022 Jun 5. Inorg Chem. 2022. PMID: 35658408 Free PMC article.
-
Depth of α-synuclein in a bilayer determined by fluorescence, neutron reflectometry, and computation.Biophys J. 2012 Feb 8;102(3):613-21. doi: 10.1016/j.bpj.2011.12.051. Epub 2012 Feb 7. Biophys J. 2012. PMID: 22325285 Free PMC article.
References
-
- Parkinson, J. (1817) An Essay on the Shaking Palsy (Sherwood, Neely & Jones, London).
-
- Dunnett, S. B. & Bjorklund, A. (1999) Nature 399, A32–A39. - PubMed
-
- Spillantini, M. G., Schmidt, M. L., Lee, V. M. Y., Trojanowski, J. Q., Jakes, R. & Goedert, M. (1997) Nature 388, 839–840. - PubMed
-
- Clayton, D. F. & George, J. M. (1998) Trends Neurosci. 21, 249–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

