The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells
- PMID: 15537383
- PMCID: PMC1183478
- DOI: 10.1042/BJ20041066
The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells
Abstract
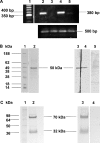
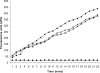
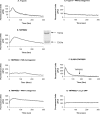
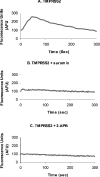
TMPRSS2 is a type II transmembrane-bound serine protease that has gained interest owing to its highly localized expression in the prostate and its overexpression in neoplastic prostate epithelium. Once activated, the serine protease domain of TMPRSS2 is released from the cell surface into the extracellular space. PAR (protease-activated receptor)-2 belongs to a family of G-protein-coupled receptors (PAR-1-4) that are activated by specific serine proteases, which are expressed in many normal and malignant cell types. Previous in vitro studies on prostate cancer cells suggest a role for PAR-2 in prostate cancer metastasis. A polyclonal anti-human TMPRSS2 antibody was generated against the TMPRSS2 serine protease domain. The antibody showed specific reactivity with recombinant expressed TMPRSS2, and so was used to extract and purify the cleaved active TMPRSS2 protease from prostate cancer cells. Reverse transcriptase PCR and Western blot analysis were used to show the expression of both TMPRSS2 and PAR-2 in the androgen-dependent LNCaP prostate cancer cell line. Treatment of LNCaP cells with the cellular immunopurified TMPRSS2 protease induced a transient increase in intracellular calcium, which is indicative of G-protein-coupled-receptor activation. This calcium mobilization was inhibited by cellular pre-treatment with a specific PAR-2 antagonist, but not with a PAR-1 antagonist; inhibition of the protease activity also failed to mobilize calcium, suggesting that TMPRSS2 is capable of cleaving and thereby activating the PAR-2 receptor. The calcium mobilization was also inhibited by cellular pre-treatment with suramin or 2-APB (2-aminoethoxydiphenyl borate), indicating that a G-protein pathway is involved and that subsequent calcium release is mainly from intracellular stores. The present study describes how TMPRSS2 may contribute to prostate tumour metastasis via the activation of PAR-2.
Figures
References
-
- Paoloni-Giacobino A., Chem H., Peitsch M. C., Rossier C., Antonarakis S. E. Cloning of the TMPRSS2 gene, which encodes a novel serine protease with transmembrane, LDLRA, and SRCR domains and maps to 21q22.3. Genomics. 1997;44:309–320. - PubMed
-
- Del Rosso M., Fibbi G., Pucci M., D'Alessio S., Del Rosso A., Magnelli L., Chiarugi V. Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin. Exp. Metastasis. 2002;19:193–207. - PubMed
-
- Cocks T. M., Moffatt J. D. Protease-activated receptors: sentries for inflammation? Trends Pharmacol. Sci. 2000;21:103–108. - PubMed
-
- Hooper J. D., Clements J. A., Quigley J. P., Antalis T. M. Type II transmembrane serine proteases: insights into an emerging class of cell surface proteolytic enzymes. J. Biol. Chem. 2001;276:857–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

