Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells
- PMID: 15537713
- PMCID: PMC527137
- DOI: 10.1073/pnas.0407643101
Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells
Abstract
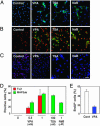
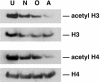
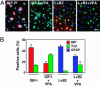
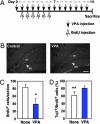
It has become apparent that chromatin modification plays a critical role in the regulation of cell-type-specific gene expression. Here, we show that an inhibitor of histone deacetylase, valproic acid (VPA), induced neuronal differentiation of adult hippocampal neural progenitors. In addition, VPA inhibited astrocyte and oligodendrocyte differentiation, even in conditions that favored lineage-specific differentiation. Among the VPA-up-regulated, neuron-specific genes, a neurogenic basic helix-loop-helix transcription factor, NeuroD, was identified. Overexpression of NeuroD resulted in the induction and suppression of neuronal and glial differentiation, respectively. These results suggest that VPA promotes neuronal fate and inhibits glial fate simultaneously through the induction of neurogenic transcription factors including NeuroD.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

