Nonassociative learning processes determine expression and extinction of conditioned fear in mice
- PMID: 15537742
- PMCID: PMC534706
- DOI: 10.1101/lm.86104
Nonassociative learning processes determine expression and extinction of conditioned fear in mice
Abstract
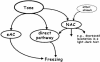
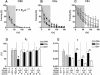
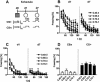
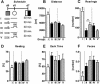
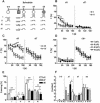
Freezing to a tone following auditory fear conditioning is commonly considered as a measure of the strength of the tone-shock association. The decrease in freezing on repeated nonreinforced tone presentation following conditioning, in turn, is attributed to the formation of an inhibitory association between tone and shock that leads to a suppression of the expression of fear. This study challenges these concepts for auditory fear conditioning in mice. We show that acquisition of conditioned fear by a few tone-shock pairings is accompanied by a nonassociative sensitization process. As a consequence, the freezing response of conditioned mice seems to be determined by both associative and nonassociative memory components. Our data suggest that the intensity of freezing as a function of footshock intensity is primarily determined by the nonassociative component, whereas the associative component is more or less categorical. We next demonstrate that the decrease in freezing on repeated nonreinforced tone presentation following conditioning shows fundamental properties of habituation. Thus, it might be regarded as a habituation-like process, which abolishes the influence of sensitization on the freezing response to the tone without affecting the expression of the associative memory component. Taken together, this study merges the dual-process theory of habituation with the concept of classical fear conditioning and demonstrates that sensitization and habituation as two nonassociative learning processes may critically determine the expression of conditioned fear in mice.
Figures



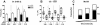



References
-
- Blair, H.T., Schafe, G.E., Bauer, E.P., Rodrigues, S.M., and LeDoux, J.E. 2001. Synaptic plasticity in the lateral amygdala: A cellular hypothesis of fear conditioning. Learn. Mem. 8: 229-242. - PubMed
-
- Blanchard, R.J., Griebel, G., Henrie, J.A., and Blanchard, D.C. 1997. Differentiation of anxiolytic and panicolytic drugs by effects on rat and mouse defense test batteries. Neurosci. Biobehav. Rev. 21: 783-789. - PubMed
-
- Boulis, N.M. and Davis, M. 1989. Footshock-induced sensitization of electrically elicited startle reflexes. Behav. Neurosci. 103: 504-508. - PubMed
-
- Cain, C.K., Blouin, A.M., and Barad, M. 2003. Temporally massed CS presentations generate more fear extinction than spaced presentations. J. Exp. Psychol. Anim. Behav. Process 29: 323-333. - PubMed
-
- Cordero, M.I. and Sandi, C. 1998. A role for brain glucocorticoid receptors in contextual fear conditioning: Dependence upon training intensity. Brain Res. 786: 11-17. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
